A Scalable Balz-Schiemann Reaction Protocol in a Continuous Flow Reactor
Summary
A detailed scalable continuous flow protocol is presented to synthesize an aryl fluoride from an aryl amine through the Balz-Schiemann reaction.
Abstract
The demand for aromatic fluorides is steadily increasing in the pharmaceutical and fine chemical industries. The Balz-Schiemann reaction is a straightforward strategy for preparing aryl fluorides from aryl amines, via the preparation and conversion of diazonium tetrafluoroborate intermediates. However, significant safety risks exist in handling the aryl diazonium salts when scaling up. In order to minimize the hazard, we present a continuous flow protocol that has been successfully performed at a kilogram scale that eliminates the isolation of aryl diazonium salts while facilitating efficient fluorination. The diazotization process was performed at 10 °C with a residence time of 10 min, followed by a fluorination process at 60 °C with a residence time of 5.4 s with about 70% yield. The reaction time has been dramatically reduced by introducing this multi-step continuous flow system.
Introduction
The Balz−Schiemann reaction is a classic method for replacing the diazonium group with fluorine by heating ArN2+BF4− without a solvent1,2. The reaction can be applied to a wide variety of aryl amine substrates, making it a generally applicable approach to synthesize aryl amines, which are frequently utilized for advanced intermediates in pharmaceutical or fine chemical industries2,3. Unfortunately, harsh reaction conditions are often employed in the Balz-Schiemann reaction, and the reaction generates potentially explosive aryldiazonium salts4,5,6,7,8. Other challenges associated with the Balz-Schiemann reaction are the formation of side products during the thermal decomposition process and its modest yield. In order to minimize the side product formation, thermal dediazotization can be performed in nonpolar solvents or using neat diazonium salts9,10, which means the aryldizanium salts should be isolated. However, the diazotization of aromatic amines is generally exothermic and fast, which is a risk associated with the isolation of the explosive diazonium salt, especially in large-scale production.
In recent years, continuous flow synthesis technologies have helped to overcome the safety issues associated with the Balz-Schiemann reactions11,12. Although there are some examples of diazotization of aromatic amines using continuous microreactors for deamination at positions para to aryl-chlorides, 5-azodyes, and chlorosulfonylation, these contributions were only reported on a laboratory scale13,14,15,16,17. Yu and co-workers developed a continuous kilo-scale process for the synthesis of aryl fluorides18. They have shown that the improved heat and mass transfer of a flow system would benefit both the diazotization process and the fluorination process. However, they used two separate continuous flow reactors; therefore, the diazotization and thermal decomposition processes were investigated separately. A further contribution was published by Buchwald and co-workers19, where they presented a hypothesis that if the product formation was proceeding through the SN2Ar or SN1 mechanism, then the yield may be improved by increasing the concentration of the fluoride source. They developed a flow-to-continuous stirred tank reactor (CSTR) hybrid process in which the diazonium salts were generated and consumed in a continuous and controlled manner. However, the heat and mass transfer efficiency of a CSTR is not good enough as a tube flow reactor, and a large CSTR cannot be expected to be used with explosive diazonium salts in large-scale production. Subsequently, Naber and co-workers developed a fully continuous flow process to synthesize 2-fluoroadenine from 2,6-diaminopurine20. They found that the exothermic Balz-Schiemann reaction was easier to control in a continuous flow manner and that the tubing dimensions of the flow reactor would influence the heat transfer and temperature control aspects – a tube reactor with large dimensions shows a positive improvement. However, the tube reactor's scaled-up effect will be notable, and the poor solubility of the polar aryl diazonium salt in organic solvents is troublesome for static tube reactors, which face a blockage risk. Even though remarkable progress has been established, there are still some problems associated with large-scale Balz-Schiemann reactions. Thus, the development of an improved protocol that would provide rapid and scalable access to aryl-fluorides is still significant.
The challenges associated with large-scale Balz−Schiemann reaction processing include the following:(i)the thermal instability of an accumulated diazonium intermediate over a short time period21; (ii) the long processing times; and (iii) the non-uniform heating or the presence of water in the diazonium fluoroborate, leading to uncontrollable thermal decomposition and increased by-product formation22,23. Additionally (iv) in some flow processing modes, an isolation of the diazonium intermediate is still required due to its low solubility14, which is then fed into a uncontrolled rate decomposition reaction. The risk of handling a large quantity of in-line diazonium salt cannot be avoided. Thus, there is significant benefit in developing a continuous flow strategy to solve the abovementioned problems and avoid both the accumulation and the isolation of the unstable diazonium species.
In order to establish an inherently safer production of chemicals in pharmaceuticals, our group has focused on multi-step continuous flow technology. In this work, we apply this technology to the Balz−Schiemann synthesis on a kilogram scale in a way that eliminates the isolation of aryl diazonium salts, while facilitating efficient fluorination.
Protocol
CAUTION: Carefully check the properties and toxicities of the chemicals described here for the appropriate chemical handling of the relevant material as per the material safety data sheets (MSDS). Some of the chemicals used are detrimental to health, and special care must be taken. Avoid inhalation and contact with skin of these materials. Please wear the proper PPE during the whole process.
1. Preparation of feeds for continuous flow protocol
- Purchase BF3·Et2O with a concentration of 8.1 mmol/mL. Label the glass bottle with 2.5 kg of BF3·Et2O as Feed A.
- Prepare a solution of substrate 1 as Feed B. Add 12.7 L of tetrahydrofuran (THF) to a clean 50 L vessel with a mechanical stirrer. Start the stirrer at 150 rpm, and then add 2-Methylpyridin-3-amine (0.5 kg) carefully to the above vessel. Visually check for complete dissolution. Then stop the stirrer and transfer the solution into a container and label as Feed A.
NOTE: Ensure that the water content on the Karl Fischer (KF) reaction of THF is below 0.5% w/w. The water content influences the generation of by-products, such as hydrolyzed OH Imp-1; therefore, anhydrous THF was used. If the water content of the reaction mixture is over 1%, the by-product percentage will increase up to 5%. THF with a <0.5% water content is a normal standard, not strictly for the anhydrous THF standard. - Prepare a solution of tert-butyl nitrite as Feed C. Add 10.7 L of THF to a clean 50 L vessel with a mechanical stirrer. Start the stirrer with moderate rpm and add tert-butyl nitrite (0.53 kg) to the above vessel. Stir for 10 min. Then transfer the solution into a container and label as Feed C.
- Label a container with 25 L heptane as Feed D.
NOTE: Ensure that the water content on the KF reaction of heptane is below 0.5%. There are two roles that heptane plays in this protocol: i) to dilute the diazonium salt slurries, which can slow down the gas stream during the diazonium decomposition process; and ii) to remove nonpolar impurities in the distillation process during the first-time phase separation. - Label a container with 2 L of THF as Feed E, which will be used as washing solution.
2. Continuous flow equipment setup
- Prepare two modules of a microflow reactor with 9 mL of internal reaction volume, one dynamic mixing tube reactor with 500 mL of internal reaction volume, one constant flow pump with a PTFE pump head, and three constant flow pumps with a 316 L pump head.
- Assemble the equipment according to the process flow sheet shown in Figure 1. Check the mechanical integrity of all the connections between pumps, pipelines, and flow reactors before use.
- For the pumps, set up the following flow rates: pump A at 23.8 mL/min; pump B at 3.4 mL/min; pump C at 22.8 mL/min; and pump D at 50 mL/min.
- Maintain the temperature regulation by setting the jacket outlet temperature of the premixing and diazonium salt formation zone at -5 °C and the jacket outlet temperature of the thermal decomposition zone at 60 °C.
- For an equipment safety check and a leak-test, perform the following steps.
- Place the dosing pipelines of pumps A, B, C, and D into the Feed E bottle. Place the discharging pipeline into the waste collecting bottle.
- Start the pumps A, B, C, and D . Regulate the back pressure up to 3 bars, slowly.
- Observe the stability of each pump, and check all the joints, pipelines, and reactors for any solvent leakage.
- Observe the jacket inlet and outlet temperature of each zone and the real-time inlet pressure of each pump and check whether they are within the target ranges.
- Stop the pumps A, B, C, and D after 10 min of steady state equilibrium.
3. Continuous flow reaction processing
- Place dosing pipelines A, B, C, and D into pumps A, B, C, and D, respectively. Place the discharging pipeline into the waste collecting bottle.
- Start pumps A and B simultaneously and record the time. Start pump C after 30 s and pump D after 8 min.
- Place the discharging pipeline into the product collecting vessel after 10 min of steady state equilibrium.
- Observe and record the temperature of each zone and the pressure of each pump.
- Place dosing pipeline B into Feed E at the completion of Feed B pumping.
- Place the discharging pipeline into the waste collecting bottle. Place dosing pipelines A, C, and D into the Feed E bottle.
- Stop pumps A, B, C, and D after 10 min of the washing process.
4. Distillation of organic solvents
- Adjust the pH value to 1-2 by adding 4 M HCl into the product collecting vessel at 20-30 °C.
- Separate the aqueous layer to an interim vessel.
NOTE: After adding 4 M HCl to adjust the pH value, there are two layers in the vessel. The product was acidified in hydrochloride salt form, which can be dissolved in the bottom aqueous layer, while some nonpolar impurities were dissolved in the upper heptane layer. - Adjust the pH value of the above separated aqueous layer to 9-10 by adding 20% NaOH aqueous at 20-30 °C.
- Add tert-butyl methyl ether (5.4 L) to the above vessel.
- Stir the mixture for 10 min before letting the mixture stand for another 10 min.
- Partition the mixture between the organic layer and the aqueous layer. Collect the organic layer into a container and discharge the aqueous layer into the separator vessel.
- Add ter-butyl methyl ether (4.6 L) to the separator vessel.
- Stir the mixture for 10 min before letting the mixture stand for another 10 min.
- Partition the mixture between the organic layer and the aqueous layer. Keep the organic layer in the separator vessel and collect the aqueous layer in the waste container.
- Add the first part of the separated organic layer into the separator vessel.
- Wash the combined organic phase with 4% citric acid to pH 4-5.
- Partition the above mixture and transfer the organic layer to distillation equipment.
- Distill the organic solvents at 1 atm and 60 °C, and then vacuum distill (25 mmHg) at 60 °C to obtain the product.
Representative Results
The model reaction is shown in Figure 2. 2-Methylpyridin-3-amine (compound 1 in Figure 2) was chosen as the starting material to prepare 2-methylpyridin-3-fluoride (compound 3 in Figure 2) via the Balz-Schiemann reaction. The experimental parameters were systematically investigated by varying reaction temperature and residence time. Feed A is 0.35 M 2-methylpyridin-3-amine in THF. Feed B is pure BF3·Et2O at an 8.1 M concentration. Feed A and Feed B were first introduced into the premixing and cooling microflow module. Then, Feed C was charged into the dynamically mixed flow reactor to merge with premixed compound 1 and BF3·Et2O for a generation of diazonium salts. The solid was sufficiently dispersed to a slurry state in the dynamic tube reactor without tube blocking. The molar ratio of compound 1:BF3·Et2O: tert-butyl nitrite is 1:3.4:1.2. Finally, the diazonium slurry was fed into the thermal decomposition microflow reactor. Feed D was charged to the thermal decomposition zone at the same time as the diazonium slurry. The overall mixture was gathered into the product collecting vessel. Further purification was conducted in a batch manner.
Compared with the batch results (shown in Table 3), the reaction temperature of the flow experiments was successfully controlled at 10 °C while obtaining the crude product with >70% HPLC purity (Table 1, Entry 5), which was higher than what was obtained in batch processing (Table 3 and Supplementary File 1). The major impurities generated in the reaction are the hydrolyzed product/aryl hydroxide called imp-1 and the reduced material called imp-2 (Supplementary File 1 and Supplementary File 2). It is common to form imp-1 from moisture in the reaction system. Therefore, the specification of the water content of the solvent and the co-solvent was set below 0.5%. A typical experimental process batch process was the reaction of diazonium formation carried out in dry THF. To a stirred solution of 1 (10 g, 89.0 mmol) in THF (150 mL) at -10 °C under argon, BF3·Et2O (38.18 g, 266 mmol) was added dropwise. After 0.5 h, t-BuNO2 (10.17 g, 98 mmol) was added dropwise while maintaining an internal temperature of -20 to -10 °C. Solid precipitates were formed after 1 h. The solid was slowly transferred to 150 mL of n-heptane at 60 °C (safety note: Avoid isolation of the solid; experiments on a small scale revealed that it is unstable at 0 °C to room temperature). The residue was partitioned between organic solvents and HCl (1 M aq., 50 mL). The organic layer was washed with water, dried over MgSO4, and concentrated. Nearly 6.1 g of reddish-brown oily liquid was obtained with a 60% yield, analyzed by MS, 1H NMR, and 9F NMR (Supplementary File 3, Supplementary File 4, and Supplementary File 5).
Nearly 98% of conversion was reached within the 10 min residence time when the flow rate is 50 mL/min (sum of feed A to C; Table 2, Entry 2). Increasing the flow rate from 50 mL/min to 100 mL/min will lead to a lot of starting material remaining in the reaction (Table 2, Entry 3). Lowering the flow rate can lead to full consumption of the starting material (Table 2, Entry 1), but the production efficiency will be restricted. Therefore, a flow rate of 50 mL/min is chosen for scale up production with a daily capacity of 72 L/day.
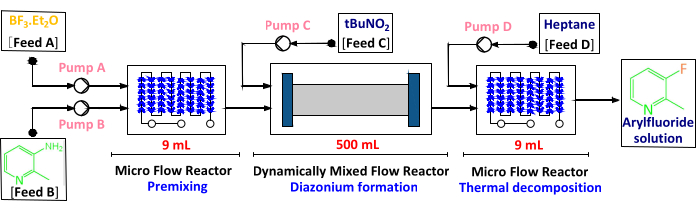
Figure 1: The continuous flow processing flowchart. There are three zones: premixing, diazonium formation, and thermal decomposition zone. The internal volume of each reactor is 9 mL, 500 mL, and 9 mL, respectively. Please click here to view a larger version of this figure.
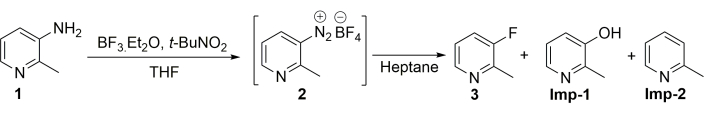
Figure 2: The synthesis of 2-methylpyridin-3-fluoride from 2-metyhlpyridin-3-amine via the Balz-Schiemann reaction. There are two major impurities generated in the process, one is the byproduct of hydrolysis imp-1 and the other is a reduced byproduct imp-2. Please click here to view a larger version of this figure.
| Entry | Diazotization zone | Thermal decomposition zone | Conversion of 1 | HPLC purity of 3 | ||
| Flow rate (mL/min) | Residence time (s) | Flow rate (mL/min) | Residence time (s) | |||
| 1 | 33 | 909 | 66 | 8.20 | 100.00% | 69.35% |
| 2 | 50 | 600 | 100 | 5.40 | 98.13% | 70.63% |
| 3 | 100 | 300 | 200 | 2.70 | 56.30% | 45.64% |
Table 1: The temperature effect of the diazonium formation process. The standard flow rates of pumps A, B, C, and D are 23.8 mL/min, 3.4 mL/min, 22.8 mL/min, and 50.0 mL/min, respectively. The residence time are listed as follows: a premixing zone of 20 s, a diazonium formation zone of 600 s, and a thermal decomposition zone of 5.4 s. The reaction temperature of thermal decomposition is 60 °C.
| Process manner | Diazonium formtion temperature (°C) | Batch size | Conversion of 1 | HPLC Purity of 3 |
| Batch process | -20 | 10 g | 100.00% | 66.62% |
| Flow process | 10 | 0.5 kg | 98.00% | 70.63% |
Table 2: The residence time effect of the diazotization and thermal decomposition processes. The flow rate at the diazotization zone is the sum of Feeds A to C. The standard reaction temperature is 0 °C. The internal volume of the diazotization zone is 500 mL, and of the thermal decomposition zone is 9 mL.
| Entry | Inner Temperature (°C) | HPLC Purity (%) | ||||
| 1 | 3 | Imp-1 | Imp-2 | Unknown Imp-3 | ||
| 1 | -20 | 1.84 | 60.80 | 9.98 | 0.00 | 1.53 |
| 2 | -10 | 0.53 | 58.56 | 6.17 | 0.00 | 2.08 |
| 3 | 0 | 0.00 | 70.99 | 9.16 | 1.12 | 1.31 |
| 4 | 5 | 0.06 | 64.85 | 0.00 | 3.54 | 6.74 |
| 5 | 10 | 0.00 | 70.63 | 0.00 | 0.00 | 5.84 |
| 6 | 20 | 0.00 | 56.07 | 0.00 | 0.00 | 5.22 |
Table 3: Comparison between batch and flow processes. The IPC results are shown in Supplementary File 1 and Supplementary File 2. Abbreviations: IPC = In-process control, HPLC = High performance liquid chromatography, Imp = Impurity.
Supplementary File 1: The HPLC IPC results of fluorination in the batch process. Please click here to download this File.
Supplementary File 2: The HPLC IPC results of fluorination in the flow process. Please click here to download this File.
Supplementary File 3: The MS spectrum of 3. MS (ESI), m/z calculated – 112.05 (M+H)+ and detected – 112.07. Please click here to download this File.
Supplementary File 4: The 1H NMR spectrum of 3. 400 MHz in CDCl3: δ8.32 (dt, J = 4.8, 1.5 Hz, 1H), 7.34 (ddd, J = 9.5, 8.2, 1.4 Hz, 1H), 7.20-7.09 (m, 1H), 2.55 (d, J = 3.0 Hz, 3H). Please click here to download this File.
Supplementary File 5: The 19F NMR of spectrum of 3. 376.5 MHz in CDCl3: δ-124.10. Please click here to download this File.
Discussion
A continuous flow protocol of the Balz-Schiemann reaction has been successfully performed through a combination of a micro-channel flow reactor and a dynamically mixed flow reactor. This strategy features several advantages compared with the batch process: (i) it is safer with controlled diazonium salt formation; (ii) it is more amenable to a higher reaction temperature, 10 °C versus -20 °C; and (iii) it is more efficient without isolation of the diazonium intermediate, two steps in one continuous process. Specifically, the continuous flow equipment setup process is critical for this protocol, because besides the chemistry transformation, the reliability of a flow process is mainly dependent on the stability of the equipment. In brief, this protocol provides a technique for use with a wide range of aryl amine substrates, which are not feasible to diazotize and further react under batch conditions.
There are some experiences to be shared about flow technology development after this scalable Balz-Schiemann continuous flow project. When considering how to develop flow processes, the solid formation is an important parameter. In this situation, the following strategies could be tried. Firstly, (i) modify the protocol in batch-mode to maintain a homogenous phase throughout the reaction via changing reagents, solvents, or the temperature (ii) or choose a suitable flow reactor that allows for processing of slurries such as a dynamically mixed flow reactor. Another situation is that the feed of raw materials is a slurry. Normally, it is difficult to pump fine suspensions consistently and effectively in a scaled-up flow production. Pay attention to the pump’s ability to pump viscous liquid while handling a pressure drop. In addition, accumulation of particles in the reactor, especially in the microflow reactor, can lead to blockage, and ultimately failure. Therefore, combinations of flow reactor types with complementary advantages need to be considered to match the chemical attributes when carrying out continuous flow processing. Indeed, that is the driver, in this example, for the choice of two reactor types to realize this continuous flow process, which can be called the hybridized flow process.
There are still some limitations of this protocol. The heat and mass transfer efficiency of a dynamically mixed flow reactor is not as good as the microflow reactor. There will be a medium scaled-up effect when continuing to increase the flow rate. There are no flow rate monitors in this protocol, which risks an un-matched material ratio if the pump has trouble without notification.
Generally, the flow chemistry process has been shown to be superior to the batch process for synthetic transformations in the following situations: (i) using explosive or highly reactive reagents with a large safety risk (i.e., avoiding a lot of active material in the reactor); (ii) generating highly active or unstable intermediates (i.e., avoiding a long holding time before quenching); (iii) needing precise temperature control (i.e., avoiding nonuniform thermal transfer); and (iv) involving multi-liquid phase reactions (i.e., requiring enhanced mixing to accelerate the reaction rate). For those batch processes that can be transferred to the flow process, there are the benefits of increased product quality and reproducibility via enhanced and precise control of the process parameters.
Flow technology has opened new avenues for chemical pathways. Even though it may not be the universal solution to solve all the production problems that were non-viable in the batch process, from an environmental, safety and financial standpoint, it is a powerful tool to conduct multi-step processes effectively for high value-added chemicals in the pharmaceutical and fine chemical industries.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank the support of Shenzhen Science and Technology Program (Grant No. KQTD20190929172447117).
Materials
| 2-Methylpyridin-3-amine | Raffles Pharmatech Co. Ltd | C2021236-SM5-H221538-008 | HPLC: >98%, Water by KF ≤0.5% |
| 316L piston constant flow pump | Oushisheng (Beijing) Technology Co.,Ltd | DP-S200 | |
| BF3.Et2O | Whmall.com | B802217 | |
| Citric acid | Titan Technology Co., Ltd | G83162G | |
| con.HCl | Foshang Xilong Huagong | 1270110101601M | |
| Dynamically mixed flow reactor | Autichem Ltd | DM500 | 316L reator with 500 mL of internal volume |
| Heptane | Shenzhen Huachang | HCH606 | Water by KF ≤0.5% |
| Micro flow reactor | Corning Reactor Technology Co.,Ltd | G1 Galss AFR | Glass module with 9 mL of internal volume |
| PTFE piston constant flow pump | Sanotac China | MPF1002C | |
| Sodium hydroxide | Foshang Xilong Huagong | 1010310101700 | |
| tert-Butyl methyl ether | Titan Technology Co., Ltd | 01153694 | |
| tert-Butyl nitrite | Whmall.com | XS22030900060 | |
| Tetrahydrofuran | Titan Technology Co., Ltd | 1152930 | Water by KF ≤0.5% |
References
- Alexander, J. C., Stephen, G. D., Paul, M. R., James, E. T. Beyond the Balz-Schiemann reaction: The utility of Tetrafluoroborates and Boron Trifluoride as nucleophilic fluoride sources. Chemical Reviews. 115 (2), 566-611 (2014).
- Mo, F., Qiu, D., Zhang, L., Wang, J. Recent development of Aryl Diazonium chemistry for the derivatization of aromatic compounds. Chemical Reviews. 121 (10), 5741-5829 (2021).
- Riccardo, P., Maurizio, B., Alessandra, P. Flow chemistry: Recent developments in the synthesis of pharmaceutical products. Organic Process Research & Development. 20 (1), 2-25 (2016).
- Ball, N. D., Sanford, M. S. Synthesis and reactivity of a Mono-σ-aryl Palladium(iv) fluoride complex. Journal of the American Chemical Society. 131 (11), 3796-3797 (2009).
- Griffete, N., Herbst, F., Pinson, J., Ammar, S., Mangeney, C. Preparation of water-soluble magnetic nanocrystals using aryl diazonium salt chemistry. Journal of the American Chemical Society. 133 (6), 1646 (2011).
- Stefan, A., Gunther, S., Matthew, J. F., Heinz, S. A one-pot Diazotation-Fluorodediazoniation reaction and fluorine gas for the production of fluoronaphthyridines. Organic Process Research & Development. 18 (8), 993-1001 (2014).
- Carl, T., Alexandre, L., Rajeev, S. B., Réjean, R. Concise and efficient synthesis of 4-Fluoro-1H-pyrrolo[2,3-b]pyridine. Organic Letters. 5 (26), 5023-5025 (2003).
- Nicolas, O., Erwan, L. G., François, X. F. Handling diazonium salts in flow for organic and material chemistry. Organic Chemistry Frontiers. 2 (5), 590-614 (2015).
- Fortt, R., Wootton, R., Mello, A. D. Continuous-flow generation of anhydrous diazonium species: Monolithic microfluidic reactors for the chemistry of unstable intermediates. Organic Process Research & Development. 7 (5), 762-768 (2003).
- Liu, Y., Zeng, C., Wang, C., Zhang, L. Continuous diazotization of aromatic amines with high acid and sodium nitrite concentrations in microreactors. Journal of Flow Chemistry. 8 (3-4), 139-146 (2018).
- Arlene, B., Aisling, L., Alex, C. P., Marcus, B. Forgotten and forbidden chemical reactions revitalised through continuous flow technology. Organic & Biomolecular Chemistry. 19 (36), 7737-7753 (2021).
- Jianli, C., Xiaoxuan, X., Jiming, L., Zhiqun, Y., Weike, S. Revisiting aromatic diazotization and aryl diazonium salts in continuous flow: highlighted research during 2001-2021. Reaction Chemistry & Engineering. 7 (6), 1247-1275 (2022).
- Li, B., Widlicka, D., Boucher, S., Hayward, C., Young, J. Telescoped flow process for the syntheses of N-Aryl pyrazoles. Organic Process Research & Development. 16 (12), 2031-2035 (2012).
- Zhi, Y., Yan, L., Chuan, Y., Wei-ke, S. Continuous flow reactor for Balz-Schiemann reaction: a new procedure for the preparation of aromatic fluorides. Tetrahedron Letters. 54 (10), 1261-1263 (2013).
- Li, B., Steven, G. Development of flow processes for the syntheses of N-aryl pyrazoles and diethyl cyclopropane-cis-1,2-dicarboxylate. Acs Symposium. 1181 (14), 383-402 (2014).
- Zhiqun, Y., Hei, D., Xiaoxuan, X., Jiming, L., Weike, S. Continuous-Flow diazotization for efficient synthesis of Methyl 2-(Chlorosulfonyl)benzoate: An example of inhibiting parallel side reactions. Organic Process Research & Development. 20 (12), 2116-2123 (2016).
- Jiming, L., et al. Continuous-flow double diazotization for the synthesis of m-difluorobenzene via Balz-Schiemann reaction. Journal of Flow Chemistry. 10 (4), 589-596 (2020).
- Zhiqun, Y., Yanwen, L., Chuanming, Y. A Continuous kilogram-scale process for the manufacture of o-Difluorobenzene. Organic Process Research & Development. 16 (10), 1669-1672 (2012).
- Hathaniel, H. P., Timothyl, J. S., Stephen, L. B. Rapid synthesis of aryl fluorides in continuous flow through the Balz-Schiemann reaction. Angewandte Chemie International Edition. 55 (39), 11907-11911 (2016).
- David, R. S., François, L., William, J. M., John, R. N. An improved Balz-Schiemann reaction enabled by ionic liquids and continuous processing. Tetrahedron. 75 (32), 4261-4265 (2019).
- He, G., Wang, D., Liang, C., Chen, H. Theoretical study on thermal safety of preparing fluorobenzene by the Balz-Schiemann reaction and fluorodenitration reaction. Journal of Chemical Health & Safety. 20 (1), 30-34 (2013).
- Schotten, C., Leprevost, S. K., Yong, L. M., Hughes, C. E., Browne, D. L. Comparison of the thermal stabilities of diazonium salts and their corresponding triazenes. Organic Process Research & Development. 24 (10), 2336-2341 (2020).
- Sharma, Y., Nikam, A. V., Kulkarni, A. A. Telescoped sequence of exothermic and endothermic reactions in multistep flow synthesis. Organic Process Research & Development. 23 (2), 170-176 (2018).

