Collecting Marine Gnathiid Isopod Fish Parasites with Light Traps
Summary
We present a method to collect marine gnathiid isopod fish parasites using light traps placed at field sites via breath-hold diving or scuba diving.
Abstract
A method to collect marine gnathiid isopod fish parasites with the use of light traps is presented. Gnathiid isopods are a major group of marine fish parasites that feed on blood and fluid from host fishes, mostly at night. Like ticks and mosquitos on land, they associate only temporarily with their host and spend most of their life free-living in the benthos. Given their high mobility and transient and predominantly nocturnal association with hosts, they cannot easily be collected by capturing free-living hosts. However, they are readily attracted to underwater light sources, creating the opportunity to collect them in light traps. Here the design and individual steps involved in the deployment and processing of specially adapted light traps for collecting free-living stages of gnathiid isopods are outlined. Sample results and possible modifications of the basic protocol for a variety of different sampling needs are presented and discussed.
Introduction
Parasitic crustaceans are important in the ecology and life histories of reef fishes. The biomass and energy they remove from their hosts are considerable and influence behavior, physiology, and survivorship1. Gnathiid isopod crustaceans represent the most prominent group of fish parasites in tropical and subtropical reef systems, where they are both abundant and diverse2,3 and are the primary food item of cleaner fishes4,5. Gnathiids are generally 1-3 mm in size. They have unusual life histories in which only the three juvenile stages feed on the blood and body fluids of fishes6,7. They are most active at night8,9, and while vision appears to play some role, host-finding10 relies heavily on olfactory cues to find hosts11,12. Each of the three juvenile feeding stages feeds on a single host fish, with each feed separated by a molting phase. After the final feed, third-stage larvae metamorphose into non-feeding adults, which reproduce and then die. Given that feeding requires only brief association with the host, while each inter-feeding interval lasts days, gnathiids spend most of their life free-living in the benthos.
Gnathiids impact hosts in multiple ways1. Aside from their role as drivers of interactions between cleaner fish and clients13,14,15, gnathiids can increase cortisol levels and decrease hematocrit in adult fish hosts16 and in high numbers, can even cause death17. For juvenile fish, even a single gnathiid can be fatal18,19,20, and even if the fish survives, its ability to compete for space and escape predators is compromised20,21,22. Avoiding gnathiids may even constitute one of the benefits of nocturnal migration in some reef fishes23.
In addition to cleaner fishes, gnathiid populations can be impacted by other micro carnivorous fishes24, as well as corals25,26. Ocean warming and the associated loss of live corals appear to have opposing impacts on gnathiids27,28,29.
Given their clear ecological importance and the likely influence of anthropogenic environmental change on their populations, there are compelling reasons to include them in ecological studies of coral reefs. However, their unique life history and the small number of researchers who study them create a barrier to the development, implementation, and dissemination of reliable, reproducible sampling methods to collect them for research.
Light traps have long been used to collect small marine organisms at night30,31. They take advantage of and are based on the fact that many nocturnally active organisms, including arthropods, are attracted to light. Traditionally they have been used to collect planktonic organisms in the water column30. However, the basic principles can be applied to collecting free-swimming organisms that are active near the benthos. Here we present a light trapping method adapted for collecting free-living stages of gnathiid isopods near the ocean bottom in remote coral reef environments such as the Philippines. For collecting in remote areas, these light traps (Figure 1) offer some advantages over other methods developed for collecting these organisms32. They are highly portable and durable, requiring only three parts, which are easily obtainable and inexpensive. They are also negatively buoyant, as when deployed, they are completely filled with seawater. Because they depend on light for attraction, they are only effective at night for collecting nocturnally active species. They also attract more than the target species, requiring sorting of the samples under a dissecting scope to obtain the target organisms. Three methods have thus far been used by our team and collaborators to collect gnathiids in coral reef systems throughout the world32. These include emergence traps, live fish-baited traps, and light traps, each with advantages and limitations.
Protocol
Collection of samples was permitted by the Department of Agriculture-Bureau of Fisheries and Aquatic Resources (0154-18 DA-BFAR) in accordance with Philippine laws and regulations (RA 9147; FAO 233) and approved by the Silliman University (SU) animal ethics committee.
1. Light Traps
- Construction
- Construct light traps from commercial polyvinyl chloride (PVC) tubes originally designed for plumbing. Use 10-15 cm diameter PVC cut to 30-40 cm length (Figure 1).
- To both ends of the tubes, add PVC "caps" with a transparent acrylic funnel inserted in the center of the opening and glue in place with transparent epoxy glue (Figure 1). Let it dry.
- Ensure that one end of the tube has a screw-on or otherwise removable lid and that both ends are water-tight when the trap is "closed" (e.g., with the addition of an O-ring).
- Light source
- Before deployment, turn on an underwater light/torch (see Table of Materials) and place it in the tube, facing one of the transparent funnels, such that the light from the underwater torch illuminates the area in front of one side of the tube. If necessary, chemical glow sticks can be used in place of underwater torches, although their light intensity is lower.
NOTE: The light attracts a variety of small nocturnal organisms31, including gnathiids, and drives them to swim into the tube through the clear funnel. Once they have entered the tube, they are unable to escape due to the geometry of the light trap (small funnel opening) and the continued presence of a light source.
- Before deployment, turn on an underwater light/torch (see Table of Materials) and place it in the tube, facing one of the transparent funnels, such that the light from the underwater torch illuminates the area in front of one side of the tube. If necessary, chemical glow sticks can be used in place of underwater torches, although their light intensity is lower.
- Placement
- When in the water at the deployment site, fill light traps, with the light turned on, with seawater, and secure both ends. To ensure that the torch is not below or blocking the funnel tip, tilt the "front" of the tube upward to allow the torch to slide back away from the funnel.
- Place traps on the seafloor, in the sand or rubble, next to coral heads or other complex structures known to attract fish. Focus the light cone "inwards", towards areas where fish aggregate.
NOTE: In shallow water, traps can be placed by breath-hold diving. Deeper deployment requires scuba.
- Retrieval
- Immediately prior to retrieving the trap, seal the openings of both the funnels (on either end of the tube) with a piece of modeling clay or rubber stopper sealing, keeping all the seawater and the contained organisms inside.
NOTE: The organisms will remain in the trap once the batteries of the lights have expired and the light is no longer illuminated. This provides flexibility when the traps are retrieved ("soak time"). Factors to consider when deciding on soak time are presented below (see Discussion).
- Immediately prior to retrieving the trap, seal the openings of both the funnels (on either end of the tube) with a piece of modeling clay or rubber stopper sealing, keeping all the seawater and the contained organisms inside.
- Transport
- Once the traps have been retrieved from the bottom, carry them to a boat, or swim ashore.
- Maintain traps close to ambient seawater temperature once removed from the ocean.
- Transport them to the laboratory for processing as soon as possible since no gas or water exchange will take place once removed from the ocean.
2 Laboratory processing
- Storing and filtering of the samples
- Once the light traps are removed from the ocean and brought back to the lab, empty their contents into buckets with fresh seawater.
- Add aeration to keep organisms alive until filtering.
- Filter the contents of the bucket by pouring through a funnel lined with 50-100 µm plankton mesh, then empty the contents into a 100 mL container of fresh seawater.
- Use a pipette to draw from this smaller container to place aliquots of the sample into a Petri dish for microscopy. Repeat until the entire sample has been processed.
- Gnathiid isopod identification and rearing
- Because light trap samples attract multiple species of small invertebrates, carefully screen the samples to identify and remove gnathiid isopods. 10-20x magnification is the best for this task (Figure 2).
NOTE: Identifying gnathiids at the family level does not require living specimens. However, adult gnathiids, which are seldom caught in light traps, are needed for morphological species identification and breeding (see reference1,3,9 for a methodology for breeding and rearing gnathiids in captivity). - In cases where gnathiids need to be kept alive for rearing, gently remove them with a pipette and place them in small plastic containers of fresh seawater.
- Because light trap samples attract multiple species of small invertebrates, carefully screen the samples to identify and remove gnathiid isopods. 10-20x magnification is the best for this task (Figure 2).
Representative Results
For sampling in the central Philippines, the outlined trap design (Figure 1) was used. When 36 traps were set overnight (in one site), 1 to 1343 gnathiids per trap (275 ± 54) were collected. These included both fed and unfed juvenile stages (Figure 2; Table 1, 2). These results demonstrate the effectiveness of light traps for collecting gnathiid isopods under the study conditions. Figure 3 shows the placement of the trap underwater.
This method for gnathiid collection is effective and sufficiently flexible for various field sites and scientific questions. For example, reference29 used light traps to quantify the effects of coral cover and fish biomass on gnathiid abundance (Figure 4), and reference33 used similar traps to quantify the effects of hurricanes on genetic haplotypes (Figure 5). While other techniques have been developed and used for collecting gnathiids32, this technique is particularly efficient (Table 3). Emergence traps, "tents" made of plankton mesh32 require large platforms to transport, are difficult to deploy, and tear easily, requiring continual repair. Moreover, they rarely collect fed juvenile stages. Traps baited with live fish32 (necessary because gnathiids will not feed on dead fish), require capture and housing of live fish. This makes their use more difficult in remote places. Moreover, their effectiveness relies on olfactory cues11,12, which are influenced by the size of the fish, the size of the openings of the trap, and water currents. They tend to collect fewer gnathiids, are more difficult to retrieve than light traps, and only collect fed stages.

Figure 1: Light trap PVC tubes. Right: top view, with the translucent funnel glued into the tube with acrylic glue, visible. Please click here to view a larger version of this figure.

Figure 2: A gnathiid isopod in a Petri dish after capture. Note the transparent body fluid of the fish and brownish red fish blood in the gnathiid's gut. Please click here to view a larger version of this figure.

Figure 3: A modified light trap (foreground) in which the light is directed down. This design can be used in calm sea conditions to sample more directly the substrate below the trap. Emergence traps are shown in the background. This figure is reprinted with permission from reference32. Please click here to view a larger version of this figure.
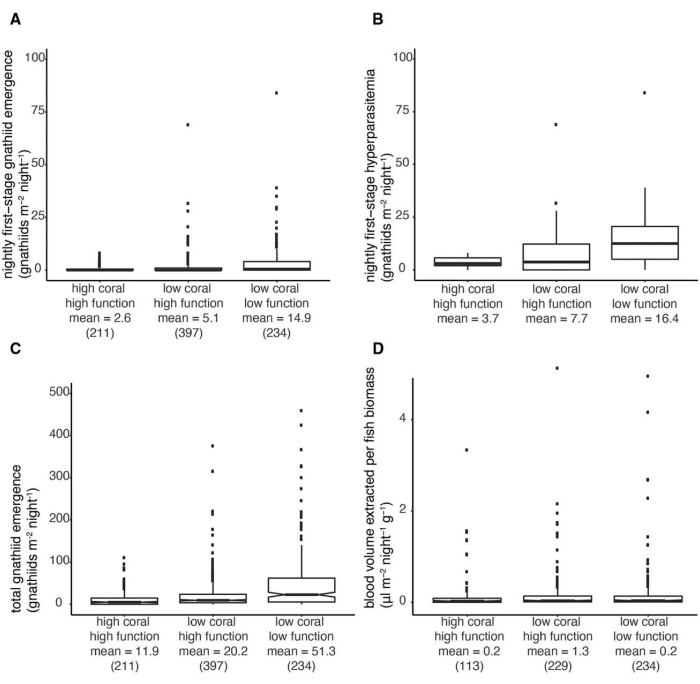
Figure 4: Boxplots showing differences in the distribution. Boxplots showing differences in the distribution (A) of first-stage gnathiid abundance, (B) first-stage hyperabundance, (C) total abundance, and (D) blood volume extracted per fish biomass. The sample size for each subgroup is shown in parentheses under each subgroup mean. Boxes display first- and third-quartile edges, while whiskers show the third quartile plus 1.5 times the interquartile range. For (C), hinges are an approximation of the 95% confidence interval. Differences seen at (A), (B), and (C) are significant; see Appendix S1: Table S7 in reference29. This figure is reprinted with permission from reference29. Please click here to view a larger version of this figure.
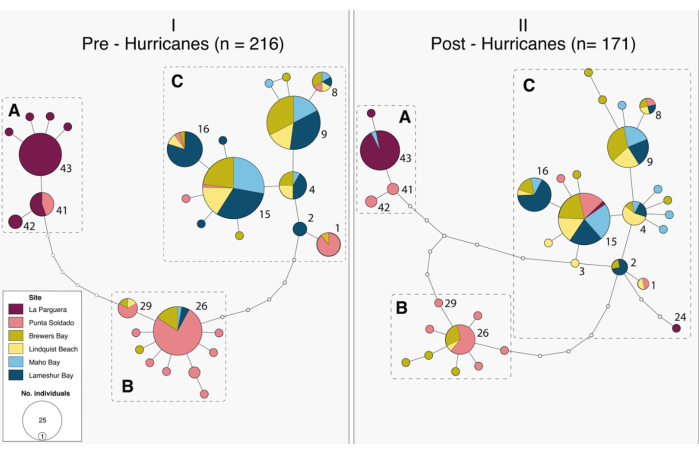
Figure 5: Haplotype networks representing the genetic diversity of Gnathia marleyi and its spatial distribution before and after the 2017 hurricanes. Pre- and post-hurricane datasets are indicated in panels I and II, respectively. The haplotypes found in both pre- and post-hurricane datasets are indicated by haplotype number. Haplogroups A, B, and C are indicated by dashed boxes. This figure is reprinted with permission from reference33. Please click here to view a larger version of this figure.
| Gnathiid count | Light traps (n) | Total count | Mean | Median | Minimum (per trap) | Maximum (per trap) | Standard Error (+-) |
| Total | 34 | 9336 | 275 | 191 | 1 | 1343 | 54 |
| Live | 34 | 6605 | 206 | 114 | 4 | 1226 | 46 |
| Dead | 34 | 2667 | 86 | 42 | 1 | 659 | 24 |
Table 1: Summary statistics for gnathiid counts from 36 light traps deployed by breath-hold diving overnight in the Bantayan coral reef, Philippines, from July – September 2017. The numerical value in the Live column refers to gnathiids from the light trap that were alive at the time of counting, the Dead column refers to gnathiids that were dead, and the Total column is the sum total of the dead and live gnathiid counts.
| Gnathiid count | N | Total count | Mean |
| Total | 10 | 434 | 43 |
Table 2: Summary statistics for gnathiid counts from light traps deployed for 3 hours in Bantayan coral reef, Negros Oriental province, Philippines, from July-August 2022.
| Trap type | Median count | 95% CI | |
| Lower | Upper | ||
| Un-baited Emergence | 0.31 | 0.04 | 0.81 |
| Fish-baited Emergence | 0.42 | 0.19 | 0.69 |
| Fish-baited Tripod | 0.92 | 0.46 | 1.46 |
| Open-mesh Fish-baited | 1.5 | 0.35 | 3.54 |
| Lighted Plankton Trap | 5.69 | 2.69 | 9.58 |
Table 3: The performance of different trap designs, including light traps, in the Caribbean were compared. Estimates of median count per sample and 95% confidence intervals for each trap design were evaluated in the multi-trap comparison. Estimates were derived from 10,000 bootstrapping iterations drawing with replacement from the 26 sample counts for each trap type. This table is adapted with permission from reference32.
Discussion
Traditional light traps, such as those used for collecting larval fishes, are large and are suspended in water column34. In contrast, the light traps described here are small and deployed on the sea bottom. These traps can be easily transported and rapidly deployed. They can be placed by breath-hold (free)-diving in shallow sites (as in this study) or on scuba in deeper sites, and attract both fed and unfed
juvenile stages.
Variations of the benthic light traps described here for studying gnathiids have been used for a variety of purposes by our team and collaborators, and modifications can be made in the design or protocol, depending on the specific purpose (Figure 3). These are described below.
Some studies require live gnathiids. These include studies identifying the source (fish species) of blood meals35,36, or studies that require rearing. The light traps in this study were 30 cm long, with a 10.16 cm diameter, and hence a volume of ~1.3 L. When they become overly crowded with organisms, the oxygen demand inside the tube can surpass the delivery of oxygen via the ports, causing organisms to die and exacerbating the problem. To avoid this, the trap can be deployed for shorter periods or modified by increasing its volume (larger diameter PVC) or adding additional holes covered with plankton mesh9,29,32. Note that while larger diameter PVC can be used to increase the volume, this is done at the cost of lower portability, resulting in the transport of fewer traps to the field site. This is of particular concern when the transport involves extended surface swims and/or the use of small boats with limited storage space.
Alternatively, or in addition, the trap can be deployed for a shorter duration. A major advantage of this design is that while the trap will only collect organisms at night (when the light is effective), it can be set at any time and retrieved at any time. To collect as many gnathiids as possible, we set traps before sunset and retrieved them shortly after sunrise the following day. However, to maximize the number of live gnathiids, we limit deployment to 3-4 hours of darkness. A short set time also reduces the amount of "bycatch" that must be sorted through to extract the gnathiids.
The traps described here lie horizontally, with the beam of the light pointing in one direction. Thus, the stimulus signal extends as far as the light will shine, usually traversing multiple substrate types, and some light is emitted from the rear funnel as well. In order to focus sampling on a particular substrate at a short distance from the trap, the trap can be modified to sit vertically, with the light pointing downward (Figure 3). This is accomplished by adding "legs" to form a tripod29,32. However, this type of light trap is useful only in low-current situations.
The torch we used (see materials table) takes four (4) AAA batteries. We use rechargeable batteries to reduce cost and waste. The burn time for these lights is approximately 12 hours, which allows them to function overnight. However, a suitable substitute is "glowsticks". These are particularly useful when setting in deeper water, such as mesophotic reefs.
While the light traps described here attract a wide range of small, mobile invertebrates, the composition of this bycatch is highly variable. However, when set in a habitat that includes rubble and fish, gnathiids are caught consistently. Although this demonstration study focused on sites in the Philippines, traps of similar design have also been used successfully for studies at sites in the Caribbean region and the Great Barrier Reef.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Funding was provided by the US National Science Foundation (NSF OCE 2023420 and DEB 2231250, P. Sikkel PI). We thank the municipality of Dumaguete City, Negros Oriental, Philippines, for permission to conduct this study. We also thank the many volunteers for their field assistance and the staff and our colleagues at the Silliman University Institute for Environmental and Marine Sciences for their support.
Materials
| Buckets, small sample containers | hardware store | ||
| Funnels | Supplier No. 2209-03 | Funnels: AMERICAN SCIENTIFIC LLC SE - 75 mm (3”) | https://us.vwr.com/store/product/8884369/plastic-funnels |
| Main body of light traps (made from commercially available PVC sanitarty pipes) | (SKU 145640) | Alasco Sanitary uPVC Pipes Series 1000 107mm/4' | https://alascopvcpipes.com/product/alasco-standard-sanitary-upvc-pipe-series-1000/. This brand can be found in the Philippines. Other simular brands can also be used |
| Modeling clay | Can be found in art suppliy and childreans toy stores | To seal the funnel after retreival | |
| Plankton mesh (50-100 µm) | any reputable brand and source | https://www.adkinstruments.in/products/plankton-nets-in-various-mesh-size-1633936883 | |
| Screw on lids for the light trap | Alasco Sanitary Clean-Out 4" | https://alascopvcpipes.com/product/alasco-standard-sanitary-upvc-clean-out/. This brand can be found in the Philippines. Other simular brands can also be used | |
| Scuba/snorkel equipment | any reputable brand and source | ||
| Stereo-microscopes | Scientific suppliers | ||
| Underwater touches | Princeton Tec Ecoflare or Fantasea Nanospotter 6023 |
References
- Sikkel, P. C., Welicky, R. L. The ecological significance of parasitic crustaceans. Parasitic Crustacea. 17 (17), 421-477 (2019).
- Svavarsson, J., Bruce, N. L. New gnathiid isopod crustaceans (Cymothoida) from Heron Island and Wistari Reef, southern Great Barrier Reef. Zootaxa. 4609 (1), 4609 (2019).
- Shodipo, M. O., Sikkel, P. C., Smit, N. J., Hadfield, K. A. First record and molecular characterisation of two Gnathia species (Crustacea, Isopoda, Gnathiidae) from Philippine coral reefs, including a summary of all Central-Indo Pacific Gnathia species. International Journal for Parasitology: Parasites and Wildlife. 14, 355-367 (2021).
- Losey, G. S. Cleaning symbiosis in Puerto Rico with comparison to the tropical Pacific. 4 (4), 960-970 (1974).
- Grutter, A. S., et al. Parasite infestation increases on coral reefs without cleaner fish. Coral Reefs. 37, 15-24 (2018).
- Smit, N. J., Davies, A. J. The curious life-style of the parasitic stages of gnathiid isopods. Advances in Parasitology. 58. , 289-391 (2004).
- Tanaka, K. Life history of gnathiid isopods-current knowledge and future directions. Plankton and Benthos Research. 2 (1), 1-11 (2007).
- Sikkel, P. C., Schaumburg, C. S., Mathenia, J. K. Diel infestation dynamics of gnathiid isopod larvae parasitic on Caribbean reef fish. Coral Reefs. 25, 683-689 (2006).
- Santos, T. R. N., Sikkel, P. C. Habitat associations of fish-parasitic gnathiid isopods in a shallow reef system in the central Philippines. Marine Biodiversity. 4, 83-96 (2019).
- Nagel, L. The role of vision in host-finding behaviour of the ectoparasite Gnathia falcipenis (Crustacea). Isopoda). Marine and Freshwater Behaviour and Physiology. 42 (1), 31-42 (2009).
- Sikkel, P. C., Sears, W. T., Weldon, B., Tuttle, B. C. An experimental field test of host-finding mechanisms in a Caribbean gnathiid isopod. Marine Biology. 158, 1075-1083 (2011).
- Vondriska, C., Dixson, D. L., Packard, A. J., Sikkel, P. C. Differentially susceptible host fishes exhibit similar chemo-attractiveness to a common coral reef ectoparasite. Symbiosis. 81 (3), 247-253 (2020).
- Grutter, A. S. Parasite infection rather than tactile stimulation is the proximate cause of cleaning behaviour in reef fish. Proceedings of the Royal Society of London. Series B: Biological Sciences. 268 (1474), 1361-1365 (2001).
- Sikkel, P. C., Cheney, K. L., Côté, I. M. In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Animal Behaviour. 68 (2), 241-247 (2004).
- Sikkel, P. C., Herzlieb, S. E., Kramer, D. L. Compensatory cleaner-seeking behavior following spawning in female yellowtail damselfish. Marine Ecology Progress Series. , 1-11 (2005).
- Triki, Z., Grutter, A. S., Bshary, R., Ros, A. F. Effects of short-term exposure to ectoparasites on fish cortisol and hematocrit levels. Marine Biology. 163, 1-6 (2016).
- Hayes, P. M., Smit, N. J., Grutter, A. S., Davies, A. J. Unexpected response of a captive blackeye thicklip, Hemigymnus melapterus (Bloch), from Lizard Island, Australia, exposed to juvenile isopods Gnathia aureamaculosa Ferreira & Smit. Journal of Fish Diseases. 34 (7), 563-566 (2011).
- Grutter, A. S., Pickering, J. L., McCallum, H., McCormick, M. I. Impact of micropredatory gnathiid isopods on young coral reef fishes. Coral Reefs. 27 (3), 655-661 (2008).
- Artim, J. M., Sellers, J. C., Sikkel, P. C. Micropredation by gnathiid isopods on settlement-stage reef fish in the eastern Caribbean Sea. Bulletin of Marine Science. 91 (4), 479-487 (2015).
- Sellers, J. C., Holstein, D. M., Botha, T. L., Sikkel, P. C. Lethal and sublethal impacts of a micropredator on post-settlement Caribbean reef fishes. Oecologia. 189, 293-305 (2019).
- Allan, B. J., et al. Parasite infection directly impacts escape response and stress levels in fish. Journal of Experimental Biology. 223 (16), (2020).
- Spitzer, C. A., Anderson, T. W., Sikkel, P. C. Habitat associations and impacts on a juvenile fish host by a temperate gnathiid isopod. International Journal for Parasitology: Parasites and Wildlife. 17, 65-73 (2022).
- Sikkel, P. C., et al. Nocturnal migration reduces exposure to micropredation in a coral reef fish. Bulletin of Marine Science. 93 (2), 475-489 (2017).
- Artim, J. M., Hook, A., Grippo, R. S., Sikkel, P. C. Predation on parasitic gnathiid isopods on coral reefs: a comparison of Caribbean cleaning gobies with non-cleaning microcarnivores. Coral Reefs. 36, 1213-1223 (2017).
- Artim, J. M., Sikkel, P. C. Live coral repels a common reef fish ectoparasite. Coral Reefs. 32, 487-494 (2013).
- Paula, J. R., et al. The role of corals on the abundance of a fish ectoparasite in the Great Barrier Reef. Coral Reefs. 40, 535-542 (2021).
- Sikkel, P. C., et al. Changes in abundance of fish-parasitic gnathiid isopods associated with warm-water bleaching events on the northern Great Barrier Reef. Coral Reefs. 38 (4), 721-730 (2019).
- Shodipo, M. O., Duong, B., Graba-Landry, A., Grutter, A. S., Sikkel, P. C. Effect of acute seawater temperature increase on the survival of a fish ectoparasite. In Oceans. 1 (4), (2020).
- Artim, J. M., Nicholson, M. D., Hendrick, G. C., Brandt, M., Smith, T. B., Sikkel, P. C. Abundance of a cryptic generalist parasite reflects degradation of an ecosystem. Ecosphere. 11 (10), (2020).
- Richardson, A. J., et al. Using continuous plankton recorder data. Progress in Oceanography. 68 (1), 27-74 (2006).
- McLeod, L. E., Costello, M. J. Light traps for sampling marine biodiversity. Helgoland Marine Research. 71 (1), 1-8 (2017).
- Artim, J. M., Sikkel, P. C. Comparison of sampling methodologies and estimation of population parameters for a temporary fish ectoparasite. International Journal for Parasitology: Parasites and Wildlife. 5 (2), 145-157 (2016).
- Pagán, J. A., Veríssimo, A., Sikkel, P. C., Xavier, R. Hurricane-induced disturbance increases genetic diversity and population admixture of the direct-brooding isopod, Gnathia marleyi. Scientific reports. 10 (1), (2020).
- Doherty, P. J. Light-traps: selective but useful devices for quantifying the distributions and abundances of larval fishes. Bulletin of Marine Science. 41, 423-431 (1987).
- Jones, C. M., Nagel, L., Hughes, G. L., Cribb, T. H., Grutter, A. S. Host specificity of two species of Gnathia (Isopoda) determined by DNA sequencing blood meals. International Journal for Parasitology. 37 (8-9), 927-935 (2007).
- Hendrick, G. C., Dolan, M. C., McKay, T., Sikkel, P. C. Host DNA integrity within blood meals of hematophagous larval gnathiid isopods (Crustacea). Isopoda, Gnathiidae). Parasites & Vectors. 12 (1), 1-9 (2019).

