Versatile Technique to Produce a Hierarchical Design in Nanoporous Gold
Summary
Nanoporous gold with a hierarchical and bimodal pore size distribution can be produced by combining electrochemical and chemical dealloying. The composition of the alloy can be monitored via EDS-SEM examination as the dealloying process advances. The material’s loading capacity can be determined by studying protein adsorption onto the material.
Abstract
The potential to generate variable pore sizes, simplistic surface modification, and a breadth of commercial uses in the fields of biosensors, actuators, drug loading and release, and the development of catalysts have unquestionably accelerated the usage of nanoporous gold (NPG)-based nanomaterials in research and development. This article describes the process of the generation of hierarchical bimodal nanoporous gold (hb-NPG) by employing a step-wise procedure involving electrochemical alloying, chemical dealloying techniques, and annealing to create both macro- and mesopores. This is done to improve the utility of NPG by creating a bicontinuous solid/void morphology. The area available for surface modification is enhanced by smaller pores, while molecular transport benefits from the network of larger pores. The bimodal architecture, which is the result of a series of fabrication steps, is visualized using scanning electron microscopy (SEM) as a network of pores that are less than 100 nm in size and connected by ligaments to larger pores that are several hundred nanometers in size. The electrochemically active surface area of the hb-NPG is assessed using cyclic voltammetry (CV), with a focus on the critical roles that both dealloying and annealing play in creating the necessary structure. The adsorption of different proteins is measured by solution depletion technique, revealing the better performance of hb-NPG in terms of protein loading. By changing the surface area to volume ratio, the created hb-NPG electrode offers tremendous potential for biosensor development. The manuscript discusses a scalable method to create hb-NPG surface structures, as they offer a large surface area for the immobilization of small molecules and improved transport pathways for faster reactions.
Introduction
Often seen in nature, hierarchical porous architectures have been imitated at the nanoscale to alter the physical characteristics of materials for improved performance1. Interconnected structural elements of various scales of length are a characteristic of the hierarchical architecture of porous materials2. Dealloyed nanoporous metals typically have unimodal pore size distributions; hence, multiple techniques have been devised to produce hierarchically bimodal porous structures with two separate pore size ranges3. The two fundamental objectives of the material design approach, namely the large specific surface area for functionalization and rapid transport pathways, which are distinct and inherently in conflict with one another, are fulfilled by functional materials possessing structural hierarchy4,5.
Performance of the electrochemical sensor is determined by the electrode morphology, since the nanomatrix’s pore size is crucial for molecular transport and capture. Small pores have been found to aid in target identification in complicated samples, whereas bigger pores enhance the target molecule’s accessibility, increasing the sensor’s detection range6. The template-based fabrication, electroplating, bottom-up synthetic chemistry, thin film sputtering deposition7, complex flexible matrices based on polydimethylsiloxane support8, alloying of various metals followed by selective etching of the less noble metal, and electrodeposition are some of the methods that are frequently used to introduce nanostructures into the electrode. One of the best methods for creating porous structures is the dealloying procedure. Due to the disparity in dissolution rates, the sacrificial metal, which is the less noble metal, significantly influences the final morphology of the electrode. An interconnected network of pores and ligaments results from the effective process of creating nanoporous gold (NPG) structures, in which the less noble component selectively dissolves out of the starting alloy, and the remaining atoms reorganize and consolidate9.
The method of dealloying/plating/re-dealloying used by Ding and Erlebacher to make these nanostructures involved first subjecting the precursor alloy composed of gold and silver to chemical dealloying using nitric acid, followed by heating at a higher temperature with a single pore size distribution to create the upper hierarchical level, and removing the remaining silver using a second dealloying to produce the lower hierarchical level. This method was applicable to thin films10. Using ternary alloys, which are comprised of two comparatively more reactive noble metals that are eroded away one at a time, was advised by Biener et al; Cu and Ag were initially removed from the Cu-Ag-Au material, leaving behind bimodally structured, low-density NPG samples11. Long-range ordered structures are not produced by the procedures outlined utilizing ternary alloys. Bigger pores were produced by extracting away one of the phases of the master alloy of Al-Au employed by Zhang et al., which produced the bimodal structure with a minimal degree of order12. An ordered hierarchical structure has reportedly been created by controlling several length scales, through the use of processing pathways that include disassembling bulk materials and putting basic components together into larger structures. In this case, a hierarchical NPG structure was made via direct ink writing (DIW), alloying, and dealloying13.
Here, a two-step dealloying method for fabricating a hierarchical bimodal nanoporous gold (hb-NPG) structure employing various Au-Ag alloy compositions is presented. The amount of reactive element below which dealloying stops is, in theory, the parting limit. The surface diffusion kinetics is slightly impacted by the parting limit or dealloying threshold, which is typically between 50 and 60 atomic percentage for electrolytic dissolution of the more reactive component from a binary alloy. A large atomic fraction of Ag in the Au:Ag alloy is necessary for the successful synthesis of hb-NPG, since both the electrochemical and chemical dealloying processes cannot be successfully completed at low concentrations near the parting limit14.
The benefit of this method is that the structure and pore size can be tightly controlled. Each step in the protocol is crucial for fine-tuning the typical porosity length scale and the typical distance between ligaments15. To regulate the rate of ion interfacial diffusion and dissolution, the applied voltage is carefully calibrated. To prevent cracking during dealloying, the Ag dissolution rate is controlled.
Protocol
1. Constructing a coating of nanoporous gold with hierarchical bimodal architecture on gold wires – Alloying
- Assemble an electrochemical cell in a 5 mL beaker. Use a Teflon-based lid with three holes to contain the three-electrode setup.
NOTE: Teflon is a popular material for making lids, as it does not react with other chemicals. - Place a platinum wire counter-electrode, an Ag/AgCl (saturated KCl) reference electrode, and a gold wire with a diameter of 0.2 mm and a length of 5.0 mm functioning as a working electrode (see Table of Materials) conveniently in each hole of the lid. Maintain a distance of 0.7 cm between the working electrode and the counter electrode.
NOTE: The length of the wire is 1 cm, and after clipping the alligator clip, the exposed part going into the solution is 0.5 cm. The remaining portion is covered using polytetrafluoroethylene tape (see Table of Materials). This length is precisely measured using a scale each time the experiment is performed. The gold wire is cleaned in several steps before being used in the study; It is first immersed in concentrated nitric acid, then rinsed and dipped in piranha solution, and finally immersed in sodium borohydride. These strong reagents aid in eliminating any impurities that might be adhering to the wire. - Prepare a 50 mM solution each of K[Ag(CN)2] and K[Au(CN)2] (see Table of Materials) in water. Add 0.5 mL of K[Au(CN)2] solution and 4.5 mL of K[Ag(CN)2] salt solution in the 5 mL beaker.
CAUTION: Be careful when handling and disposing of cyanide salts. Wear protective gloves, appropriate clothing, respiratory protection, and eye protection while handling it, and work in a fume hood. Cyanide salt solutions must be collected after the experiment is over and placed in a separate, clearly marked waste container. When in contact with acids, hazardous fumes are released. To preserve the ionic mobility, 0.25 M Na2CO3 is added to the stock 40 mM solutions of each of the two salts. - Mix the solution thoroughly by inserting a magnetic stirrer bar into the electrochemical cell, at a constant stirring speed of 300 rpm.
NOTE: Verify that the stirrer bar is clean. Aqua regia can be used to clean it if there are contaminants. - Circulate argon gas through the solution using a 1/32 inch (in) inner diameter, 5/32 in outer diameter, and 1/16 in wall thickness silicone tubing (see Table of Materials), going inside the electrochemical cell to take out any dissolved oxygen in the electrolyte solution.
NOTE: The leak in the setup is avoided by making it tight enough. - Connect the potentiostat using alligator clips that are clipped to the appropriate electrodes once the electrochemical cell is assembled completely.
NOTE: It is best to label the clips with the name of the electrode they will be attached to in order to avoid confusion. - Use the software (PowerSuite; see Table of Materials) to perform electrodeposition utilizing chronoamperometry after turning on the potentiostat. Configure the software using the desired parameters. For 600 s, the potential is timed with a fixed value of -1.0 V15.
NOTE: Let the booting process finish on its own before specifying the parameters. Supplementary File 1 shows screenshots for the use of this specific software. - Press run and choose external cell to complete the alloy deposition on the working electrode. A thick white coating is seen at the end of the process.
NOTE: Ensure the connections are secure and the solution is stirring steadily. If everything works as it should, the current oscillates between 300 and 400 µA.
2. Constructing a coating of nanoporous gold with hierarchical bimodal architecture on gold wires – Dealloying
NOTE: This phase of the protocol is based on the partial dealloying of the alloyed wires.
- Configure the electrochemical cell once more, as in the prior process (step 1.2), in which three electrodes are kept 0.7 cm from each other in an electrolyte solution. Use 4 mL of 1 N nitric acid as the electrolyte solution for partial dealloying.
NOTE: Because the electrolyte is diluted, it does not completely remove the less noble element, which here is silver. - Leave the stirrer bar running to evenly circulate the solution at a constant speed of 300 rpm.
- Once the electrochemical cell is set up, attach the potentiostat using alligator clips that are clipped to the correct electrodes.
- Employ the chronoamperometry software once more, but this time choose a potential of 0.6 V for 600 s.
- Press run, then select external cell to finish dealloying the deposited alloy on the working electrode.
NOTE: The color of the wire changes to greyish-black after this step.
3. Constructing a coating of nanoporous gold with hierarchical bimodal architecture on gold wires – Annealing
- Keep the dealloyed wires in a glass vial inside the furnace.
- Maintain the temperature in the furnace at 600 °C for 3 h.
NOTE: The temperature and duration were chosen based on optimization studies, which showed that lower temperatures did not coarsen the pores, while higher temperatures and a longer time caused cracks in the structure. - Remove the vial after the procedure is finished and the furnace is turned off. Wait till the vial cools to room temperature.
NOTE: The hot vial is removed from the furnace using a set of tongs.
4. Constructing a coating of nanoporous gold with hierarchical bimodal architecture on gold wires – Dealloying
- Immerse the partially dealloyed-annealed wires in 4 mL of concentrated nitric acid.
NOTE: When transferring the concentrated acid into the glass vial containing the wires, ensure a glass pipette is used. - Leave the partially dealloyed-annealed wires in the glass vial containing the strong nitric acid in the fume hood overnight.
NOTE: The dealloying period was kept at 24 h to allow for full dealloying. - Create the hb-NPG-coated wires the following day (Figure 1). Rinse these thoroughly with deionized water, followed by an ethanol rinse, before being used in subsequent studies. After drying, use the wires in experiments. A fresh batch of clean gold wires is used each time to prepare hb-NPG.
NOTE: Every step in the synthesis is crucial to arrive at the bimodal structure. If any of the steps are skipped, only a unimodal pore structure will result.
5. hb-NPG characterization
- Scanning electron microscope (SEM)-sample preparation
- Ensure to use a clean aluminum stub as the base for sample preparation for SEM imaging.
NOTE: The stubs are cleaned with dilute nitric acid, rinsed with ethanol, dried, and kept in a paraffin film-covered container before being used again. - Place a newly cut piece of carbon tape over the aluminum stub's flat base.
- With the aid of tweezers, cut little pieces of hb-NPG-coated wire and adhere them to the carbon tape.
NOTE: Ensure that the wire is gently gripped with tweezers to prevent the coating from coming off. - Arrange the cut pieces horizontally to reveal their morphology under a SEM (see Table of Materials); pieces arranged vertically can reveal the thickness of the material deposited on the gold wire (Figure 2).
NOTE: If the electrode has any moisture, the chamber will become contaminated, resulting in hazy images. Therefore, place the electrode in a vacuum overnight before performing SEM imaging.
- Ensure to use a clean aluminum stub as the base for sample preparation for SEM imaging.
- SEM chamber set up
- Vent the chamber using the option "vent" from the software "XT microscope" to easily open the chamber door.
NOTE: The "XT microscope" software comes with the SEM system. The chamber must first be entirely vented before the door can be smoothly opened, which typically takes 3-5 min. In adiition, the pressure of the nitrogen gas cylinder must be under 5 psi. Supplementary File 1 shows screenshots for the use of this specific software. - With the aid of a special tweezer's curved front, firmly grasp the circular aluminum stub, insert the clean sample that was previously placed on the stub into the chamber, and place it on the sample stage.
NOTE: Ensure that the stage is clean; if there are any spills, then them off with acetone and a lint-free wipe (see Table of Materials). - Select the "pump" option after placing the sample inside the chamber to produce a vacuum-like environment.
NOTE: It takes approximately 3 min to pump the chamber. Before turning on the beam, the user needs to wait for the pumping to finish. - Using the navigation camera, click on the real-time image to observe the sample's precise location.
- Vent the chamber using the option "vent" from the software "XT microscope" to easily open the chamber door.
- Imaging the sample
- Turn on the beam initially to capture an image of the sample.
NOTE: Before turning on the beam, ensure the system is vacuumed. - To bring the sample image from the navigation camera's collected image to the center of the screen and sharpen the focus, double-click on it.
- Ensure that the images are produced utilizing imaging with the compatible software and the Everhart-Thornley detector (ETD). Optimizing the spot size, scan rate, and potential is necessary.
NOTE: Starting with lower potential and spot size before moving up is always promising. - Adjust the stage distance from the beam source to 10 mm.
NOTE: To precisely measure the distance between the stage and the source; the system must be focused and connected to the Z axis. - To learn more about the architecture that is present at a lower scale, increase the magnification after obtaining a focused image at the lower magnification (Figure 3).
NOTE: Every level of the magnification increase requires focus.
- Turn on the beam initially to capture an image of the sample.
- SEM and energy dispersive spectroscopy (EDS) for elemental composition of the sample
- By choosing the color SEM option in the software, determine the elemental makeup of the material (Figure 4). It automatically assigns diverse colors to the sample's various elements. However, it is also possible to choose colors and elements manually.
NOTE: It is crucial to examine the elemental composition analysis chart and the atomic percentages of the elements to rule out choices that have an atomic percentage of 0. - By inserting the EDS detector, subject the sample under study to energy-dispersive X-ray spectroscopy. Use 15 kV potential and a spot size of 12 for collecting the EDS data.
NOTE: After pressing the insert button, the screen displays the detector inserted in real time. - Check that the program enables the creation of tiny boxes to select discrete regions on the sample image screen, to gather information for the elemental composition.
NOTE: For accurate statistics, there must be between 50 and 100,000 counts. The panel displays the overall status of the data collected. At the conclusion, a report is produced.
- By choosing the color SEM option in the software, determine the elemental makeup of the material (Figure 4). It automatically assigns diverse colors to the sample's various elements. However, it is also possible to choose colors and elements manually.
6. Cyclic voltammetry (CV) for electrochemically active surface area
- Perform the gold oxide stripping method, which involves manually setting up an electrochemical cell with the reference electrode, counter electrode, and working electrode immersed in an electrolyte.
NOTE: For the present study, Pt wire serves as the counter electrode, hb-NPG is the working electrode, and Ag/AgCl serves as the reference electrode. Sulphuric acid, 0.5 N, serves as the electrolyte. Cleaning the reference and counter electrodes in diluted nitric acid before usage is recommended. Moreover, avoid reusing the electrolyte. - Once the connections are made to the potentiostat, select the CV option from the program and configure the parameters. Fix the potential range from -0.2-1.6 V (Figure 5) and back to -0.2 V (vs. Ag/AgCl) along with a scan rate of 100 mV/s.
NOTE: No stirring is required. - Click on the run button to carry out the cyclic scan. Ensure the reverse scan reveals a noticeable peak. Using the reported conversion factor of 400 µC cm-2, integrate the charge under the peak coming from the reduction of the gold oxide monolayer to determine the electrochemical surface area of the hb-NPG wire.
NOTE: The tangent must be drawn correctly to integrate the charge under the peak properly. - Conduct an identical set of experiments on chemically dealloyed wire and after combining chemical and electrochemical dealloying on the Au:Ag (10:90) alloyed wire, to comprehend the crucial significance of dual dealloying procedures.
7. Solution depletion technique to study protein loading
- To study the real-time loading of protein molecules onto the hb-NPG surface, employ an ultraviolet-visible (UV-vis) spectrophotometer (see Table of Materials).
NOTE: In this study, protein solutions of fetuin, bovine serum albumin (BSA), and horseradish peroxidase (HRP) (see Table of Materials) were prepared separately at concentrations of 1 mg/mL, 0.5 mg/mL, and 1 mg/mL, respectively, in a 0.01 M (pH = 7.4) phosphate buffered saline (PBS) buffer. It is crucial to select a buffer and pH that maintains protein stability. - Perform baseline correction using the buffer only. Choose the software's parameters, including wavelength, time, and rate, after the correction has been made. Then, place 500 µL of the protein solution inside the cuvette.
- Ensure that the real-time protein monitoring starts after adding hb-NPG into the solution. Monitor the change in absorbance after every minute for 120 min at 280 nm after pressing the start button (Figure 6).
Representative Results
The ligament size and inter-ligament gap adjustments are of utmost significance for the manufactured electrode. Creating a structure with dual-sized pores by optimizing the Au/Ag ratios is the first step in this study, along with the characterization utilizing surface morphology, roughness factor, and loading capacity. Compared to conventional NPG, the bimodal pore structure has demonstrated a higher electrochemical surface area, roughness factor, and protein loading capacity15.
hb-NPG has demonstrated an open, linked network of ligaments and pores following chemical dealloying. Here, larger holes are indicated by an upper hierarchy, and a lower hierarchy indicates smaller pores. Figure 3 shows SEM images of the electrode with a hierarchical bimodal structure. Figure 4 illustrates color-coded elemental mapping for each step of the creation of hb-NPG, with red and yellow designating silver and gold, respectively. SEM's ability to assign distinct colors to various sections is a useful feature in the instrument.
The proportion of electrochemically active surface area (ECSA) to geometric surface area yields a roughness factor for each electrode. Utilizing the charge beneath the gold oxide reduction peak, CV was employed to assess the ECSA, and 7.64 cm2 is discovered to be the hb-NPG ECSA15.
Figure 1: Hierarchical bimodal electrode (hb-NPG) after the multistep fabrication process. The hb-NPG coating on the gold wire after the completion of alloying-dealloying-annealing-dealloying is depicted here. Please click here to view a larger version of this figure.
Figure 2: Sample preparation for SEM. Electrodes are mounted on carbon tape that is placed on aluminum stubs. The sample is then loaded into the imaging chamber. Please click here to view a larger version of this figure.
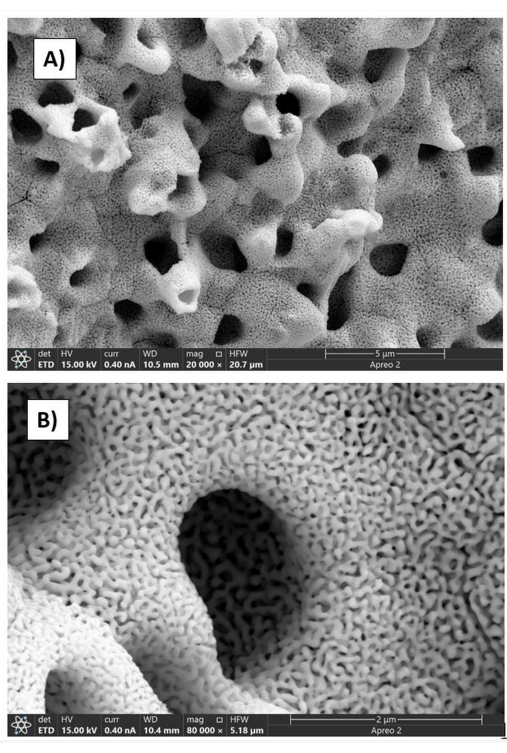
Figure 3: SEM micrograph showing the bimodal structure comprising of bigger and smaller pore sizes. Images are taken at 15 kV and a spot size of 10. (A) Upper hierarchy in the form of larger pores at 20,000x (scale bar: 5 µm). (B) Lower hierarchy depicting nanopores at a higher magnification of 80,000x (scale bar: 2 µm). This figure has been reproduced with permission from Sondhi et al.15. Please click here to view a larger version of this figure.
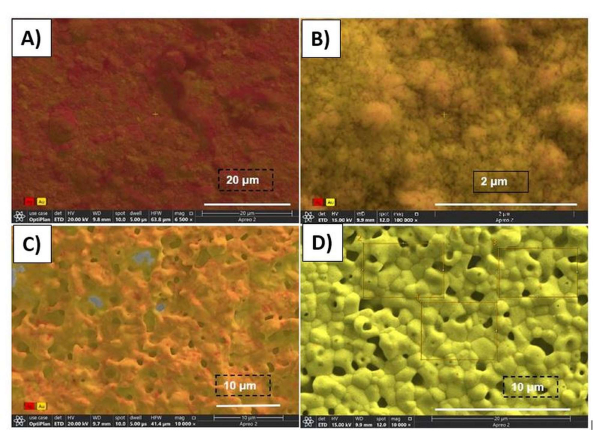
Figure 4: SEM micrographs showing the color-coded elemental mapping done after every fabrication step. SEM of the following structures with color-coded elements (Au, yellow; and Ag, red): (A) Au10:Ag90 alloy, (B) electrochemically dealloyed, (C) annealed, (D) hierarchical nanoporous structure following the last chemical dealloying step. Aux:Ag100-x stands for the alloy of gold and silver, where x is the atomic percentage of gold in the alloy. This figure has been reproduced with permission from Sondhi et al.15. Please click here to view a larger version of this figure.
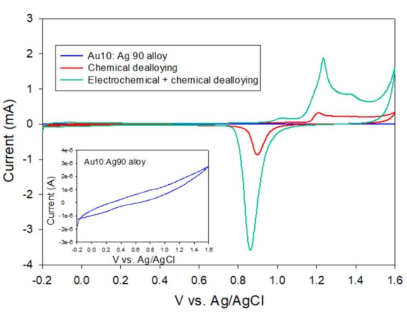
Figure 5: Cyclic voltammograms comparing the electroactive surface area of the electrodes. The curve shown as an inset (blue) depicts the CV of the Au10:Ag90 alloy. The structure created via chemical dealloying shows a small gold oxide reduction (red curve) peak. The bimodal structure incorporating chemical and electrochemical dealloying shows a much more pronounced gold oxide reduction peak (green), indicating an increase in the surface area. CV was performed using a potential scan from -0.2-1.6 V. This figure has been reproduced with permission from Sondhi et al.15. Please click here to view a larger version of this figure.
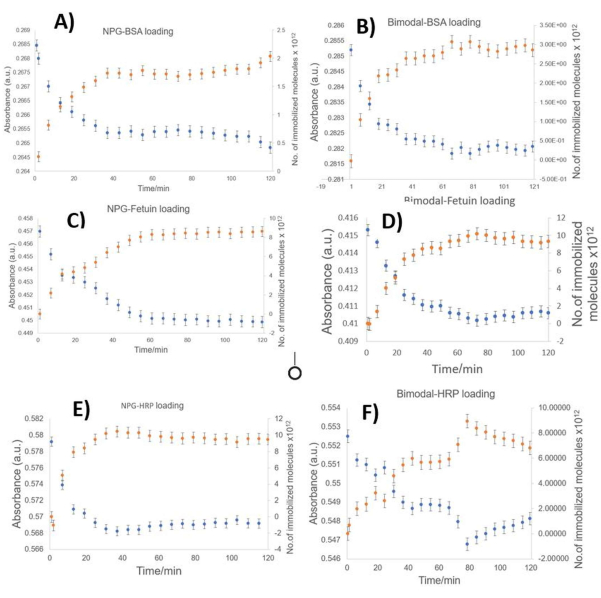
Figure 6: Absorbance versus time graph showing real-time protein loading. Real-time immobilization of BSA, fetuin, and HRP on NPG electrodes is depicted in (A), (C), and (E), while on hb-NPG is depicted in (B), (D), and (F). Change in absorbance and the number of immobilized molecules is monitored over 120 min. Absorbance was recorded after every 60 s. The average of the three readings is depicted in the graph. Please click here to view a larger version of this figure.
Supplementary File 1: User guide for the easy operation of two pieces of software used in the fabrication and characterization process of the hb-NPG. Screenshots explaning the step-by-step procedure to use "PowerSuite" and "XT microscope". Arrows used in the flowchart are pointing toward the next step of the operation. Please click here to view a larger version of this figure.
Discussion
Using a multistep procedure involving alloying, partial dealloying, thermal treatment, and acid etching, fabricating hierarchically NPG with dual-sized pores and a higher active electrochemical surface area is demonstrated.
In alloying, the standard potential of metal precursors influences how reactive they are during electrodeposition. Au and Ag ions from liquid solutions are reduced during electrodeposition16,17.
The following half-cell reactions17 depict the electrochemical dissolution of gold and silver cyanide salt solutions:
Au (CN)2– + 1e– 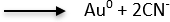
E0 = -1060 mV vs. SCE
Ag (CN)32- + 1e– 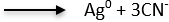
E0 = -1198 mV vs. SCE
The following parallel processes mostly determine how silver is oxidized by nitric acid in the dealloying step18.
4HNO3 + 3Ag -> 3AgNO3 + NO + 2H2O
2HNO3 + Ag -> AgNO3 + NO2 + H2O
The impact of changes in the process parameters on the electrode's electrochemical performance is well discussed. It was discovered that an NPG electrode with a bimodal pore structure, made from a gold and silver alloy with a high atomic percentage of silver and annealed at 600 °C for 3 h, contain dual-sized pores that allow for a greater protein loading than a conventional electrode with nanopores. Compared to other nanoporous electrodes, the structure's interconnected network also offers high mass transfer efficiency, improving performance in terms of activity and sensitivity15.
The interconnecting ligaments that connect the highly active, low-coordinated atoms that make the upper hierarchy of the hierarchical electrode have a dimension of 938 ± 285 nm. The electrode's application in the field of catalysis is improved by the dimensions of the ligament it produces. A ligament width of 51 ± 5 nm, a characteristic of lower hierarchical structures, enhances the electrode's capacity to immobilize bioactive compounds. The material created has a lot of potential for applications like catalysis and sensing, that need speedy detection of larger molecules as well as easy passage for those larger molecules15.
The large surface area and hierarchical structure of the electrodes have an impact on the protein loading. A greater protein loading capacity than conventional NPG has been shown for hb-NPG. Bimodal architecture provides a platform for protein interaction, communication with the substrate, and passage for the facile flow of protein molecules. The type of material and its morphology impact how many bioactive molecules can be loaded into an electrode. Because of the hierarchy, which speeds up the binding of most proteins through electrostatic and physical forces, the hb-NPG has a higher concentration of immobilized proteins15.
Even though the development of materials with complex structural designs has advanced significantly, there are still some challenges. Commercialization calls for new preparation techniques with fewer processes and cheaper production. Future research that tackles the issue of mass output and carefully analyzes the in situ development process of hierarchical structures will be interesting to work on.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by an award from the NIGMS (GM111835).
Materials
| Argon gas compressed | Fisher Scientific Compay | ||
| Bovine serum albumin (BSA) | Sigma-Aldrich (St.Louis, MO) | A9418 | > 98% purity |
| Counter electrode (Platinum wire) | Alfa Aesar | 43288-BU | 0.5 mm diameter |
| Digital Lab furnace | Barnstead Thermolyne 47,900 | F47915 | used for annealing at high temperatures |
| Digital Potentiostat/galvanostat | EG&G Princeton Applied Research | 273A | PowerPULSE software |
| Ethanol | Sigma-Aldrich (St.Louis, MO) | CAS-64-17-5 | HPLC/spectrophotometric grade |
| Fetuin from fetal calf serum | Sigma-Aldrich (St.Louis, MO) | F2379 | lyophilized powder |
| Gold wire roll | Electron Microscopy Sciences (Fort Washington, PA) | 73100 | 0.2 mm diameter, 10 ft, 99.9% |
| Hydrochloric acid | Fisher Chemical | A144C-212 | 36.5-38% |
| Hydrogen peroxide | Fisher Scientific (Pittsburg, PA) | CAS-7732-18-5 | 30% |
| Kimwipes | KIMTECH Science brand, Kimberly-Clark professional | 34120 | 4.4 x 8.2 in |
| Nitric acid | Fisher Scientific (Pittsburg, PA) | A2008-212 | trace metal grade |
| Parafilm | Bemis PM996 | 13-374-10 | 4 IN. x 125 FT. |
| Peroxidase from horseradish (HRP) | Sigma-Aldrich (St.Louis, MO) | 9003-99-0 | |
| PharMed silicone tubing | Norton | AY242606 | 1/32" Inner Diameter, 5/32" Outer Diameter, 1/16" Wall Thickness, 25' Length |
| Potassium dicyanoargentate | Sigma-Aldrich (St.Louis, MO) | 379166 | 99.96%, 10 G |
| Potassium dicyanoaurate | Sigma-Aldrich (St.Louis, MO) | 389867 | 99.98%, 1 G |
| PowerSuite software | EG&G Princeton Applied Research | comes with the instrument | |
| PTFE tape | Fisherbrand | 15-078-261 | 1" wide 600" long |
| Reference electrode (Ag/AgCl) | Princeton Applied Research | K0265 | |
| Scanning Electron Microscopy (SEM) Apreo 2C | ThermoFisher scientific | APREO 2 SEM | equipped with Color SEM technology |
| Simplicity UV system | Millipore corporation, Boston, MA, USA | SIMSV00WW | for generating Milli-Q water(18.2 MΩ cm at 25 °C) |
| Sodium Borohydride | Sigma-Aldrich (St.Louis, MO) | 213462 | 100 G |
| Sodium Carbonate | Sigma-Aldrich (St.Louis, MO) | 452882 | enzyme grade, >99%, 100 G |
| Stir bar | Fisherbrand | 14-512-153 | 5 x 2 mm |
| Sulphuric acid | Fisher Scientific (Pittsburg, PA) | A300C-212 | certified ACS plus |
| Supracil quartz cuvette | Fisher Scientific (Pittsburg, PA) | 14-385-902C | 10 mm light path, volume capacity 1 mL |
| UV-Visible Spectrophotometer | Varian Cary 50 |
References
- Fang, M., Dong, G., Wei, R., Ho, J. C. Hierarchical nanostructures: design for sustainable water splitting. Advanced Energy Materials. 7 (23), 1700559 (2017).
- Inayat, A., Reinhardt, B., Uhlig, H., Einicke, W. -. D., Enke, D. Silica monoliths with hierarchical porosity obtained from porous glasses. Chemical Society Reviews. 42 (9), 3753-3764 (2013).
- Yang, X. -. Y., et al. Hierarchically porous materials: synthesis strategies and structure design. Chemical Society Reviews. 46 (2), 481-558 (2017).
- Qi, Z., Weissmuller, J. Hierarchical nested-network nanostructure by dealloying. ACS Nano. 7 (7), 5948-5954 (2013).
- Sondhi, P., Stine, K. J. Methods to generate structurally hierarchical architectures in nanoporous coinage metals. Coatings. 11 (12), 1440-1456 (2021).
- Matharu, Z., et al. Nanoporous-gold-based electrode morphology libraries for investigating structure-property relationships in nucleic acid based electrochemical biosensors. ACS Applied Materials & Interfaces. 9 (15), 12959-12966 (2017).
- Bollella, P. Porous gold: A new frontier for enzyme-based electrodes. Nanomaterials. 10 (4), 722-740 (2020).
- Khan, R. K., Yadavalli, V. K., Collinson, M. M. Flexible nanoporous gold electrodes for electroanalysis in complex matrices. ChemElectroChem. 6 (17), 4660-4665 (2019).
- Sondhi, P., Stine, K. J. Electrodeposition of nanoporous gold thin films. in Nanofibers-Synthesis, Properties and Applications. , 1-21 (2020).
- Fujita, T. Hierarchical nanoporous metals as a path toward the ultimate three-dimensional functionality. Science and Technology of Advanced Materials. 18 (1), 724-740 (2017).
- Biener, J., et al. Nanoporous plasmonic metamaterials. Advanced Materials. 20 (6), 1211-1217 (2008).
- Zhang, Z., et al. Fabrication and characterization of nanoporous gold composites through chemical dealloying of two phase Al-Au alloys. Journal of Materials Chemistry. 19 (33), 6042-6050 (2009).
- Zhu, C., et al. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Science Advances. 4 (8), (2018).
- Artymowicz, D. M., Erlebacher, J., Newman, R. C. Relationship between the parting limit for de-alloying and a particular geometric high-density site percolation threshold. Philosophical Magazine. 89 (21), 1663-1693 (2009).
- Sondhi, P., Neupane, D., Bhattarai, J. K., Demchenko, A. V., Stine, K. J. Facile fabrication of hierarchically nanostructured gold electrode for bio-electrochemical applications. Journal of Electroanalytical Chemistry. 924, 116865 (2022).
- Cerovic, K., Hutchison, H., Sandenbergh, R. F. Kinetics of gold and a gold-10% silver alloy dissolution in aqueous cyanide in the presence of lead. Minerals Engineering. 18 (6), 585-590 (2005).
- Ciabatti, I. Gold part-ing with nitric acid in gold-silver alloys. Substantia. 3 (1), 53-60 (2019).
- Reyes-Cruz, V., Ponce-de-León, C., González, I., Oropeza, M. T. Electrochemical deposition of silver and gold from cyanide leaching solutions. Hydrometallurgy. 65 (2-3), 187-203 (2002).

