通过仪器工程自动化柑橘接穗加工,用于下游病原体检测
Summary
我们设计、制造并验证了一种快速处理富含韧皮部的树皮柑橘接穗组织的仪器。与现有方法相比,接穗组织提取器(BTE)提高了样品通量,并降低了所需的劳动力和设备成本。
Abstract
移植传播、韧皮部限制的柑橘病原体,如病毒、类病毒和细菌,是造成全球毁灭性流行病和严重经济损失的原因。例如,柑橘tristeza病毒杀死了全球超过1亿棵柑橘树,而“念珠菌亚洲自由杆菌”使佛罗里达州损失了90亿美元。使用经过病原体测试的柑橘接穗进行树木繁殖是管理此类病原体的关键。加州大学河滨分校的柑橘克隆保护计划 (CCPP) 使用聚合酶链反应 (PCR) 测定每年测试来自柑橘接穗树的数千个样品,以保护加州的柑橘并为国家清洁植物网络提供清洁繁殖装置。柑橘病毒和类病毒的高通量分子检测的一个严重瓶颈是植物组织处理步骤。
正确的组织制备对于提取优质核酸和PCR测定的下游使用至关重要。植物组织在低温下切碎、称重、冷冻干燥、研磨和离心以避免核酸降解是时间和劳动密集型的,需要昂贵且专业的实验室设备。本文介绍了一种专门用于快速处理柑橘接穗木富含韧皮部的树皮组织的仪器的验证,称为接穗组织提取器 (BTE)。与现有方法相比,BTE 可将样品通量提高 100%。此外,它还减少了劳动力和设备成本。在这项工作中,BTE样品的DNA产量(80.25 ng / μL)与CCPP的手工切碎方案(77.84 ng / μL)相当。该仪器和快速植物组织处理方案可以使加利福尼亚州的几个柑橘诊断实验室和项目受益,并成为全球其他木本多年生作物组织处理的模型系统。
Introduction
柑橘的嫁接传播限制韧皮部病原体,如类病毒、病毒和细菌,在世界每个柑橘产区都造成了毁灭性的流行病和严重的经济损失。柑橘类病毒限制了生产因素,因为它们在经济上重要的柑橘类型中引起外皮质炎和恶病质疾病,例如三叶、三叶杂交种、柑橘、柑橘和橘子 1,2,3。在加利福尼亚州,这些对类病毒敏感的柑橘类型是“易去皮者”市场增长和盈利的基础,跟随消费者对易于去皮、分段和无籽水果的偏好变化趋势4,5,6。因此,柑橘类病毒受到加州食品和农业部(CDFA)“柑橘苗圃害虫清洁计划-参议院法案140”的监管,CDFA植物害虫诊断处的实验室每年进行数千次柑橘类病毒测试7,8,9,10.自 1930 年代全球流行病开始以来,柑橘 tristeza 病毒 (CTV) 已造成超过 1 亿棵柑橘树死亡 3,9,10,11。在加利福尼亚州,病毒的茎点蚀和三叶断裂抗性对价值 36 亿美元的加州柑橘产业构成严重威胁12,13,14。因此,CDFA将CTV归类为受管制的A类植物害虫,中加州Tristeza根除局(CCTEA)的实验室每年进行广泛的实地调查和数千次病毒测试15,16。据估计,细菌“亚洲念珠菌”(CLas)和黄龙冰病(HLB)对佛罗里达州造成了近90亿美元的经济损失,原因是柑橘种植面积减少了40%,柑橘业务减少了57%,并损失了近8,000个工作岗位17,18。在加利福尼亚州,假设HLB导致的柑橘种植面积减少20%,预计将导致8,200多个工作岗位流失,该州国内生产总值减少超过五亿美元。因此,柑橘病虫害预防计划每年花费超过 4000 万美元进行调查,以测试、检测和根除来自加利福尼亚州14、17、19、20 的 CLas。
管理柑橘类病毒、病毒和细菌的一个关键要素是使用经过病原体测试的繁殖材料(即接穗)进行树木生产。经过病原体测试的柑橘接穗是在采用先进病原体消除和检测技术的综合检疫计划中生产和维护的10,21。加州大学河滨分校的柑橘克隆保护计划 (CCPP) 每年测试来自新进口到该州和美国的柑橘品种以及柑橘接穗树的数千个接穗样品,以保护加州的柑橘并支持国家柑橘清洁植物网络的功能10,17,22.为了处理大量的柑橘检测,高通量、可靠且具有成本效益的病原体检测检测是CCPP 7,10,22等项目成功的基本组成部分。
虽然聚合酶链反应(PCR)等基于分子的病原体检测分析已显著提高植物诊断实验室的通量,但根据我们的经验,实施高通量方案的最关键瓶颈之一是植物组织样品处理步骤。对于柑橘来说尤其如此,因为目前可用于处理富含韧皮部的组织(如叶柄和接穗树皮)的方案是劳动密集型的,耗时的,并且需要昂贵且专业的实验室设备。这些方案需要在低温下手工切碎、称重、冷冻干燥、研磨和离心,以避免核酸降解8,23,24。例如,在CCPP诊断实验室,样品处理包括(i)手工切碎(6-9个样品/小时/操作员),(ii)冷冻干燥(16-24小时),(iii)粉碎(30-60秒)和(iv)离心(1-2小时)。该过程还需要专门的用品(例如,重型安全锁定管,不锈钢研磨球,适配器,刀片,手套)和多件昂贵的实验室设备(例如,超低温冰箱,冷冻干燥机,组织粉碎机,液氮低温,冷冻离心机)。
与任何行业一样,设备工程和流程自动化是降低成本、提高产量以及提供高质量、统一的产品和服务的关键。柑橘行业需要低成本的组织处理仪器,这些仪器需要最少的操作技能,因此易于转移到诊断实验室和现场操作,以实现快速下游病原体检测的高样品处理能力。技术演进解决方案(TES)和CCPP开发(即设计和制造)并验证(即用柑橘样品进行测试并与标准实验室程序进行比较)一种低成本(即消除了对专业实验室设备的需求)仪器,用于快速处理富含韧皮部的柑橘组织(即接穗),称为接穗组织提取器(BTE)。 如图1所示,BTE包括一个用于电源和控制的基本组件,以及一个用于处理柑橘接穗木的可拆卸腔室。BTE腔室由一个砂轮组成,专门设计用于从柑橘接穗中剥离富含韧皮部的树皮组织。切碎的树皮组织通过滑动端口快速喷射到含有提取缓冲液的注射器中,过滤,并准备好进行核酸提取和纯化,无需任何额外的处理或制备(图1)。BTE系统还包括无纸化样品跟踪应用程序和集成称重应用程序,可将样品处理信息实时记录在在线数据库中。
BTE系统将CCPP的实验室诊断能力提高了100%以上,并始终如一地生产出适合纯化高质量核酸和使用PCR测定法检测柑橘移植传播病原体的柑橘组织提取物。更具体地说,BTE将组织处理时间从每个样品的24小时减少到~3分钟,取代了成本超过60,000美元的实验室仪器(图2,步骤2-4),并允许处理更大的样品量。
本文介绍了BTE高通量柑橘树皮组织处理、核酸提取和病原体检测验证数据,包括分别来自CCPP Rubidoux检疫设施和Lindcove基金会设施的所有适当的阳性和阴性对照。我们还介绍了与当前实验室程序相比的吞吐量和处理时间变化(图2)。此外,这项工作为柑橘病原体检测实验室提供了详细的分步协议,并展示了BTE如何支持病原体清洁苗圃,调查和根除计划的功能。
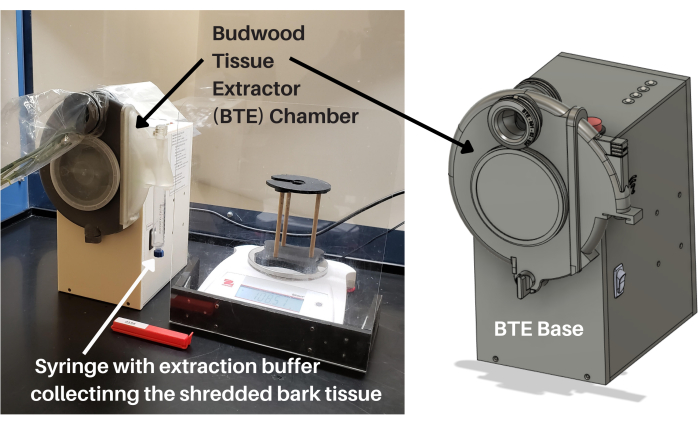
图 1:接穗组织提取器。 BTE包括一个用于电源和控制的基本组件,以及一个用于加工柑橘接穗木的可拆卸室。BTE腔室由一个砂轮组成,专门设计用于从柑橘接穗中剥离富含韧皮部的树皮组织。切碎的树皮组织通过滑口快速喷射到注射器中,过滤,并准备好进行核酸提取和纯化,无需任何额外的处理或准备。缩写:BTE = 接穗组织提取器。 请点击此处查看此图的大图。
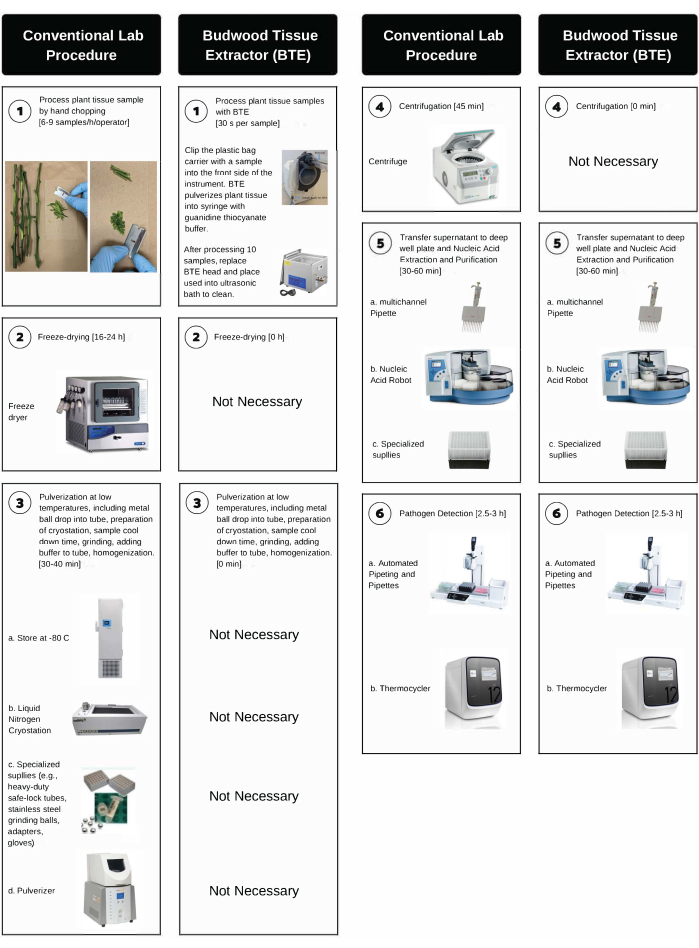
图 2:传统手工切碎实验室程序与 BTE 处理之间的分步比较。 BTE 处理涉及高通量柑橘树皮组织处理、核酸提取和病原体检测。每个步骤的时间在括号中表示。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
随着HLB柑橘病的出现,为了减少损失,柑橘行业、监管机构和诊断实验室被敦促依靠高通量核酸提取方法与低通量手动样品处理和病原体检测分析相结合,如qPCR34 ,结合疾病管理实践35对单个树木进行测试35.加州的HLB阳性率从2012年的0.01%上升到2020年的1.2%。尽管qPCR是一种强大而可靠的病原体检测工具,但目前可用的技术不允许对足够数量的植物组织进行采样和?…
Divulgations
The authors have nothing to disclose.
Acknowledgements
作者承认Cahuilla人是完成实验工作的土地的传统监护人。我们感谢加州大学河滨分校的Norman Ellstrand教授提供实验室空间,以便在UCR加州农业和食品企业(CAFÉ)倡议下为该项目开展研究活动。这项研究得到了CDFA-特种作物整笔拨款计划(拨款号18-0001-055-SC)的支持。CRB项目6100也提供了额外的支持;美国农业部国家食品和农业研究所,哈奇项目1020106;以及国家清洁植物网络-美国农业部动植物卫生检验局(AP17PPQS&T00C118,AP18PPQS&T00C107,AP19PPQS&T00C148和AP20PPQS&T00C049授予乔治斯·维达拉基斯。
Materials
| 0.08" Hex Trimmer line | PowerCare | FPRO07065 | Needed to replace blades. |
| 1 Hp, 8 gal air compressor | California Air Tools | 8010 | Quickly dry chambers after rinsed |
| 1.5 mL microcentrifuge tube | Globe Scientific | 111558B | Store sample in after swishing with syinges |
| 10 mL Syringe Set | Technology Evolving Solutions | TE006-F1-10A-G1000-E1 | Syringe material is cut into. 1 L bottle with guanidine thiocyanate buffer. WARNING – contains guanidine thiocyanate, hazardous waste service required – do not mix with bleach |
| 12" Ruler | Westcott | 16012 | To measure trimmer line before cutting |
| 12% Sodium Hypochlorite | Hasa | 1041 | Disinfects chambers after processing |
| -20 C Freezer | Insignia | NS-CZ70WH0 | Store sample after processing |
| 4" x 12" plastic bags | Plymor | FP20-4×12-10 | Bags to hold branches during shipping. O-rings attach bag to BTE chamber to seal |
| 6" Cotton Swab | Puritan | 806-PCL | Swab to remove clogs |
| 7 Gallon Storage Tote | HDX | 206152 | Holds sodium hypochlorite solution to disinfect chambers and water to rinse chambers |
| Air blow gun | JASTIND | JTABG103A | Directs air into the chambers at high pressure |
| Black Sharpie | Sharpie | S-19421 | Mark 1.5 mL tubes so you can identify sample later |
| Bottle Top Dispensor | Brand | Z627569 | Adjustable bottle top dispensor to dispense guandine into syringe |
| BTE Chamber | Technology Evolving Solutions | TE002BB-A05-E1 | Used to process budwood. Includes O-rings, BTE Slide, slide plunger, drain valve, lid, blade set, and blade set removal tool |
| Dish Soap | Dawn | 57445CT | Surfectant to improve sodium hypochlorite penetration into chamber |
| Fume hood with hepa filter | Air Science | P5-36XT-A | Fume hood with hepa filter (ASTS-030) to limit possible contamination and protect against chemical spills |
| Insulated foam shipping container | PolarTech | 261/J50C | Insulated shipping container to ship samples on ice after they are collected |
| Lab coat | Red Kap | KP14WH LN 46 | Lab coat to limit possible contamination and protect against chemical spills |
| Laptop | Microsoft | Surface | Wifi capable laptop to run TES GUI. Needed for initial setup and provides more indepth information about the tissue processing base |
| NFC Capable Phone | Samsung | Galaxy S9 | Phone to download and use TES phone app |
| NFC clip tag | Technology Evolving Solutions | TE005-Clip-E1 | Sample tag that can be linked with trees. Made to function with TES phone app |
| NFC Collar Tag | Technology Evolving Solutions | TE005-Collar-E1 | Tag that is attached to a tree. Made to function with TES phone app |
| Nitrile Gloves | Usa Scientific | 3915-4400 | Gloves to limit possible contamination and protect against chemical spills |
| Noise-Reducing Earmuff | 3M | 90565-4DC-PS | Protect ears while operating air compressor and tissue processing base |
| Polyurethane Recoil Air Hose | FYPower | 510019 | Attaches air gun to compressor |
| Saftey glasses | Solidwork | SW8329-US | Protect eyes for chemical and physical hazards |
| Spray bottle | JohnBee | B08QM81BJV | Spray bleach to deconatinate surfaces |
| Tissue Extractor Base | Technology Evolving Solutions | TE001-A-E1 | System to process plant tissue. Needs BTE or LTE chambers to function. Includes power cable, blade adapter, and 8/32" allen wrench |
| Tissue Processing Base Weight Scale | Technology Evolving Solutions | TE003-A05-200g-01-E1 | 200 g, 0.01 resolution weight scale that connects to tissue processing base to enforce weight ranges and/or link weights with sample. Includes scale, power cable, connection cable, 5ml syringe holder, tower air shield |
| Vermiculite | EasyGoProducts | B07WQDZGRP | Needed to transport hazardous waste (guanidine thiocyanate) using a hazardous waste disposal service |
| Wire Cutter | Boenfu | BOWC-06002-US | Wire cutters to cut trimmer line |
References
- Vernière, C., et al. Interactions between citrus viroids affect symptom expression and field performance of clementine trees grafted on trifoliate orange. Phytopathology. 96 (4), 356-368 (2006).
- Vernière, C., et al. Citrus viroids: Symptom expression and effect on vegetative growth and yield of clementine trees grafted on trifoliate orange. Plant Disease. 88 (11), 1189-1197 (2004).
- Zhou, C., Talon, M., Caruso, M., Gmitter, F. G., et al. Chapter 19 – Citrus viruses and viroids. The Genus Citrus. , 391-410 (2020).
- Trends and issues facing the U.S. citrus industry. Choices Magazine Online Available from: https://www.choicesmagazine.org/choices-magazine/theme-articles/trends-and-challenges-in-fruit-and-tree-nut-sectors/trends-and-issues-facing-the-us-citrus-industry (2021)
- Fruit and Tree Nuts Outlook. United States Department of Agriculture-Economic Research Service Available from: https://www.ers.usda.gov/webdocs/outlooks/98171/fts-370.pdf?v=5697 (2020)
- Forsyth, J., Fruits Damiani, J. C. i. t. r. u. s. Citrus Fruits. Types on the market. Encyclopedia of Food Sciences and Nutrition. , 1329-1335 (2003).
- Bostock, R. M., Thomas, C. S., Hoenisch, R. W., Golino, D. A., Vidalakis, G. Plant health: How diagnostic networks and interagency partnerships protect plant systems from pests and pathogens. California Agriculture. 68 (4), 117-124 (2014).
- Osman, F., Dang, T., Bodaghi, S., Vidalakis, G. One-step multiplex RT-qPCR detects three citrus viroids from different genera in a wide range of hosts. Journal of Virological Methods. 245, 40-52 (2017).
- Wang, J., et al. Past and future of a century old Citrus tristeza virus collection: A California citrus germplasm tale. Frontiers in Microbiology. 4, 366 (2013).
- Gergerich, R. C., et al. Safeguarding fruit crops in the age of agricultural globalization. Plant Disease. 99 (2), 176-187 (2015).
- Moreno, P., Ambrós, S., Albiach-Martí, M. R., Guerri, J., Peña, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Molecular Plant Pathology. 9 (2), 251-268 (2008).
- Yokomi, R. K., et al. Identification and characterization of Citrus tristeza virus isolates breaking resistance in trifoliate orange in California. Phytopathology. 107 (7), 901-908 (2017).
- Selvaraj, V., Maheshwari, Y., Hajeri, S., Yokomi, R. A rapid detection tool for VT isolates of Citrus tristeza virus by immunocapture-reverse transcriptase loop-mediated isothermal amplification assay. PLoS One. 14 (9), 0222170 (2019).
- Babcock, B. A. Economic impact of California’s citrus industry in 2020. Journal of Citrus Pathology. 9, (2022).
- Gottwald, T. R., Polek, M., Riley, K. History, present incidence, and spatial distribution of Citrus tristeza virus in the California central valley. International Organization of Citrus Virologists Conference Proceedings (1957-2010). 15, (2002).
- Yokomi, R., et al. Molecular and biological characterization of a novel mild strain of citrus tristeza virus in California. Archives of Virology. 163 (7), 1795-1804 (2018).
- Fuchs, M., et al. Economic studies reinforce efforts to safeguard specialty crops in the United States. Plant Disease. 105 (1), 14-26 (2021).
- The real cost of HLB in Florida. Citrus Industry Magazine Available from: https://citrusindustry.net/2019/07/30/the-real-cost-of-hib-in-florida/ (2019)
- McRoberts, N., et al. Using models to provide rapid programme support for California’s efforts to suppress Huanglongbing disease of citrus. Philosophical Transactions of the Royal Society B: Biological Sciences. 374 (1776), 20180281 (2019).
- Albrecht, C., et al. Action plan for Asian citrus psyllid and huanglongbing (citrus greening) in California. Journal of Citrus Pathology. 7 (1), (2020).
- Navarro, L., et al. The Citrus Variety Improvement Program in Spain in the period 1975-2001. International Organization of Citrus Virologists Conference Proceedings (1957-2010). 15 (15), (2002).
- Vidalakis, G., Gumpf, D. J., Polek, M. L., Bash, J. A., Ferguson, L., Grafton-Cardwell, E. E. The California Citrus Clonal Protection Program. Citrus Production Manual. , 117-130 (2014).
- Dang, T., Rao, A. L. N., Lavagi-Craddock, I., Vidalakis, G., et al. High-throughput RNA extraction from citrus tissues for the detection of viroids. In Viroids: Methods and Protocols. 2316, (2022).
- Osman, F., Vidalakis, G., Rao, A. L. N., Lavagi-Craddock, I., Vidalakis, G. Real-time detection of viroids using singleplex and multiplex quantitative polymerase chain reaction. Viroids: Methods and Protocols. 2316, (2022).
- Li, R., et al. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. Journal of Virological Methods. 154 (1-2), 48-55 (2008).
- Saponari, M., Manjunath, K., Yokomi, R. K. Quantitative detection of Citrus tristeza virus in citrus and aphids by real-time reverse transcription-PCR (TaqMan). Journal of Virological Methods. 147 (1), 43-53 (2008).
- Damaj, M. B., et al. Reproducible RNA preparation from sugarcane and citrus for functional genomic applications. International Journal of Plant Genomics. 2009, 765367 (2009).
- Dang, T., et al. First report of citrus leaf blotch virus infecting Bearss lime tree in California. Plant Disease. 104 (11), 3088 (2020).
- Manchester, K. L. Use of UV methods for measurement of protein and nucleic acid concentrations. BioTechniques. 20 (6), 968-970 (1996).
- Teare, J. M., et al. Measurement of nucleic acid concentrations using the DyNA QuantTM and the GeneQuantTM. BioTechniques. 22 (6), 1170-1174 (1997).
- Imbeaud, S. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Research. 33 (6), 56-56 (2005).
- Menzel, W., Jelkmann, W., Maiss, E. Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. Journal of Virological Methods. 99 (1-2), 81-92 (2002).
- Vidalakis, G., Rao, A. L. N., Lavagi-Craddock, I., Vidalakis, G., et al. SYBR Green RT-qPCR for the universal detection of citrus viroids. Viroids: Methods and Protocols. , 211-217 (2022).
- Arredondo Valdés, R., et al. A review of techniques for detecting Huanglongbing (greening) in citrus. Canadian Journal of Microbiology. 62 (10), 803-811 (2016).
- Li, S., Wu, F., Duan, Y., Singerman, A., Guan, Z. Citrus greening: Management strategies and their economic impact. HortScience. 55 (5), 604-612 (2020).
- . CDFA California Citrus Pest and Disease Prevention Program Operations Subcomittee Meeting. Meeting Minutes Available from: https://www.cdfa.ca.gov/citrus/docs/minutes/2019/OpsSubcoMinutes-11062019.pdf (2019)

