Chronic Intermittent Ethanol Vapor Exposure Paired with Two-Bottle Choice to Model Alcohol Use Disorder
Summary
Presented here is a protocol for the 2BC/CIE model of alcohol dependence in mice to study alcohol use disorder.
Abstract
Alcohol use disorder (AUD) is a chronic alcohol-related disorder that typically presents as uncontrolled drinking and preoccupation with alcohol. A key component of AUD research is using translationally relevant preclinical models. Over the past several decades, a variety of animal models have been used to study AUD. One prominent model of AUD is the chronic intermittent ethanol vapor exposure (CIE) model, which is a well-established approach for inducing alcohol dependence in rodents through repeated cycles of ethanol exposure via inhalation. To model AUD in mice, the CIE exposure is paired with a voluntary two-bottle choice (2BC) of alcohol drinking and water to measure the escalation of alcohol drinking. The 2BC/CIE procedure involves alternating weeks of 2BC drinking and CIE, which repeat until the escalation of alcohol drinking is achieved. In the present study, we outline the procedures for performing 2BC/CIE, including the daily use of the CIE vapor chamber, and provide an example of escalated alcohol drinking in C57BL/6J mice using this approach.
Introduction
Alcohol use disorder (AUD), which involves chronic excessive alcohol consumption, is one of the most common psychiatric disorders and is a global problem. AUD symptoms involve repeated cycles of intoxication, withdrawal, and cravings and are characterized by the constant consumption of alcohol without regard for the social, occupational, and health consequences1,2,3,4,5,6,7. Alcohol use disorder often occurs in conjunction with other pervasive, persistent, and impairing mental disorders8, such as ADHD9, anxiety10, or depression11 and is responsible for approximately 88,000 deaths annually in the United States alone2. Excessive or frequent alcohol use can affect a person's work status and social relationships12 and may lead to increased violence13. Physically, acute withdrawal from alcohol can result in anxiety, agitation, tremor, excessive sweating, altered consciousness, and hallucinations14,15. Furthermore, people may feel withdrawal symptoms when cutting down or stopping drinking and become irritable or cranky16. Additionally, chronic alcohol consumption can cause memory loss17 and can result in thiamine deficiency, also known as Wernicke-Korsakoff syndrome (WKS), which contributes significantly to alcohol-induced dementia18.
To further advance AUD research, it is necessary to have translationally relevant animal models of the disease. The most common model of AUD in rodents is chronic intermittent ethanol vapor exposure (CIE), which is a well-established approach for inducing alcohol dependence through repeated inhalation of alcohol vapor4,19,20,21,22,23,24,25,26,27,28,29,30. Rodent CIE procedures induce withdrawal symptoms such as handling-induced convulsions31, hyperexcitability, irritability-like behavior, anxiety-like behavior, and sleep disorders and result in an escalation of alcohol drinking22,32,33,34,35, thus meaning the CIE model has translational validity to human AUD.
In rats, the CIE model often involves the operant self-administration of alcohol to measure the escalation of intake36,37,38, whereas the mouse model involves CIE and two-bottle choice (2BC) drinking39,40. Preclinical models of alcohol dependence have consistently shown that animals increase their ethanol intake after chronic ethanol vapor exposure23,41,42,43. In mice specifically, repeated cycles of CIE have been shown to escalate voluntary ethanol intake3,21,44,45,46. Overall, prior studies demonstrate that the CIE model is sufficient to increase ethanol consumption and model AUD in rodents.
This study aims to highlight the CIE method for studying AUD and, more specifically, focus on the 2BC/CIE mouse model. We go through a detailed process of the steps necessary for performing 2BC/CIE and present an example of the escalation of alcohol drinking after CIE.
Protocol
All procedures were approved by the Purdue University Animal Care and Use Committee. The present study used 8 week old C57BL/6J mice. The CIE group had 5 mice (3 male, 2 female), and the Air group had 10 mice (5 male and 5 female). The animals were obtained from a commercial source (see Table of Materials) and were group-housed on a 12 h light-dark cycle with access to food. The body weights of the mice were measured once per week throughout the experiment.
1. General experimental design
NOTE: Two-bottle choice (2BC)/chronic intermittent ethanol vapor exposure (CIE) is a preclinical mouse model to explore ethanol dependence47.
- During the 2BC weeks, ensure that the mice have access to a bottle containing ethanol (15% w/v) and a bottle containing water for 2 h daily (Monday to Friday), beginning 2 h into the dark cycle.
- At first, allow the mice a minimum of 2 weeks of baseline 2BC drinking until a stable level of ethanol intake has been reached. Depending on the experimental needs, divide the mice into two groups (a CIE group and an Air group) or more if additional treatments are included based on the average baseline ethanol intake.
- After the baseline 2BC weeks, expose the mice from the CIE group to ethanol vapor (CIE) for four consecutive days (Monday to Thursday) with 16 consecutive hours of vapor on and 8 consecutive hours of vapor off.
NOTE: Mice from the Air group remain in their regular housing setting so they are exposed to Air with no ethanol vapor. - During the CIE/Air week, ensure 2BC does not occur. After 1 week of CIE/Air, resume the mice on 2BC for the following Monday to Friday.
- Allow mice alternate weeks of CIE/Air and 2BC for the remainder of the procedure until the escalation of alcohol drinking occurs in the CIE mice. Collect blood from the mice at least once per week of CIE to determine their blood ethanol concentration (BEC)48.
2. Experimental preparation
- Acquire all the following supplies before the start of the study: mice, cages/metal cage tops, drinking bottles to fit two per cage side by side, bedding, food, a scale, a chronic intermitted vapor chamber, and an Analox machine or another method to analyze the BECs (see Table of Materials).
- Ensure all the required chemicals are ready for the experiment: drinking water, 95% ethanol, pyrazole, heparin and sterile saline.
3. Animal habituation
- If the mice are not already housed in the same location as the experiments, allow at least 1 week for them to acclimate to the new environment. During this time, handle the mice regularly, and transfer them to any areas where experimentation will occur for 30 min daily.
- Ensure that the mice are given 1 week of two-bottle choices with both bottles filled with water to allow them to adjust to the different cage setup and being placed into an individual cage for drinking.
4. Two-bottle choice with 15% w/v ethanol and water
- Two-bottle choice habituation
- The two-bottle choice testing paradigm is the same throughout the week of two-bottle choice with water and the later testing with water and ethanol. Move the group-housed mice to their individual drinking cages 30 min before the lights turn off, and then allow the mice to drink for 2 h.
- Record the volumes of each drinking bottle before and after the 2 h drinking period. Each group undergoes 2BC for 5 days, from Monday to Friday.
NOTE: The purpose of the habituation week is to let the animals adjust to two bottles being in the cage to minimize any bias caused by the natural side position of a single bottle. During this week, both bottles are filled with water. If the supplied metal hopper is designed to hold only one bottle, carefully use the space between its bars adjacent to the first bottle to create space for the second bottle. The bottles used for drinking experiments are custom-made using 10 mL and 25 mL pipettes with the ends cut to allow a straight sipper with a ball bearing to be inserted for the mice to drink from. Using binder clips to clip the bottles to the wire lid will ensure that the bottles do not move during the drinking period (Supplementary Figure 1 and Supplementary Figure 2).
- Baseline two-bottle choice
- After habituation, allow the mice to undergo at least 2 weeks of baseline 2BC drinking to establish a stable ethanol intake. During the baseline drinking, test the mice for 2 h per day and 5 days per week (Monday to Friday) with one water bottle and a second bottle with 15% w/v ethanol. Record the volumes of each drinking bottle before and after the 2 h drinking period.
NOTE: To establish a stable baseline of ethanol intake, repeat the weeks of 2BC until each mouse has approximately less than 15% variation in intake between days. - After the mice achieve a stable baseline of ethanol intake, evenly divide the mice based on their ethanol intake into ethanol-nondependent (Air treatment) and ethanol-dependent (CIE treatment) groups. Once the baseline ethanol intake has been established, the mice undergo CIE (or Air exposure) the following week.
NOTE: Not all experiments require a non-dependent group to be included, and this must be determined based on the focus of the study. For example, ethanol-dependent mice with and without specific treatment may be a more relevant comparison.
- After habituation, allow the mice to undergo at least 2 weeks of baseline 2BC drinking to establish a stable ethanol intake. During the baseline drinking, test the mice for 2 h per day and 5 days per week (Monday to Friday) with one water bottle and a second bottle with 15% w/v ethanol. Record the volumes of each drinking bottle before and after the 2 h drinking period.
- Two-bottle choice (2BC)
- Resume the 2BC procedure as performed during baseline drinking after the first CIE week.
NOTE: The two-bottle choice mouse drinking formula is a 15% w/v ethanol mixture (1 L): 95% ethanol (195 mL) and mouse drinking water (805 mL).
- Resume the 2BC procedure as performed during baseline drinking after the first CIE week.
5. Chronic intermittent ethanol vapor (CIE) exposure
- Arrange the following materials: custom-made CIE vapor chambers (Supplementary Figure 3), 95% ethanol, an ethanol-pyrazole solution (68.1 mg/kg pyrazole mixed in 20% ethanol), a saline-pyrazole solution (68.1 mg/kg pyrazole mixed in saline), syringes (1 mL), and needles.
- Chronic intermittent ethanol vapor (CIE) procedure
- Prior to being put into the vapor chambers for CIE, intraperitoneally inject the mice with an ethanol-pyrazole solution (0.0075 mL/g), which is used to create an initial ethanol intoxication, inhibit ethanol dehydrogenase, and block the metabolism of ethanol49.
- Inject all the Air controls and other groups with the same pyrazole dose mixed with saline (0.0075 mL/g) instead of ethanol. It is recommended to give a half dose (0.00375 mL/g) of the pyrazole solutions on the first day and last day of CIE each week.
- After the injections, return the Air/control mice to their home cages, and place the cages for the CIE group inside the vapor chamber.
NOTE: During CIE, the mice are most susceptible to poor health outcomes from vapor exposure at the end of the week as they enter an extended withdrawal period; thus, a half dose of pyrazole is given on the last day of CIE to minimize the withdrawal occurring after the CIE is paused for the weekend and the following week. A half dose of pyrazole is also given each week on the first day of CIE to ease the mice back into the weekly vapor exposure. The dose of pyrazole may need to be adjusted in order to calibrate the BEC to be higher or lower, as desired. It is recommended to use a pyrazole dose ranging from 0.00375 mL/g to 0.0075 mL/g. - Once the mice are put into the vapor chambers, lock the doors, and set the pump to the appropriate vapor level (e.g., 1-3). Set the run time of the experiment. For the present study, the experiment started at 17:00 PM and finished at 09:00 AM, and a pump setting of 1-2 was used (used throughout).
- Then, press the Start button on the CIE vapor chamber control screen to start the experiment.
NOTE: This step could vary depending on the chamber being used and differences in the setup. Here, once all the mice are injected and placed into the ethanol chamber, the chamber should begin pumping 95% ethanol into a glass flask and heat the liquid ethanol into vapor. The vapor flows through the chamber where the mice are held for 16 h/day for 4 days (Monday evening through Friday morning). Each day, the mice experience 8 h of the vapor being off. The mice are then removed from the chamber at the end of the fourth day (Friday morning) and begin 2BC the following Monday. Depending on the specific vapor chamber system and setup, there may be variations in the BECs caused by the different settings/vapor levels. Various factors, such as slight differences in the system components, air flow, exhaust, etc., can cause this. Adjusting the ethanol vapor level instead of the pyrazole dose is suitable to maintain a BEC in the target range. As tolerance develops, the BECs may be too low, and the vapor level may need to be adjusted. If the gradient between the vapor levels is too steep for increasing the BECs, then adjusting the pyrazole dose accordingly is recommended to alter the BECs. - Measure the BECs (see step 5.3) of the mice at least once per week during CIE. Perform blood collection 30 min before the vapor stops to ensure the BECs have not started to drop.
NOTE: The vapor level may need to be adjusted during the experiment as ethanol tolerance builds over the course of several weeks. BECs should be tested before and after adjusting the vapor settings. - Return the mice to 2BC testing on the week following the CIE. Repeat step 4 and step 5. Keep the mice alternating between CIE and 2BC for four to eight cycles (8-16 weeks) until they have significant ethanol consumption escalation compared with their baseline drinking volume.
- Blood measurements
- Arrange the following materials: equipment to measure BECs, such as an Analox system, a blade, a tube (0.2 mL), heparin/EDTA, and a centrifuge (see Table of Materials).
- Tail-snip the CIE mice once during the CIE week, 30 min before the vapor system turns off, to collect blood for determining the BEC. The blood collection can be performed using several methods depending on the preferences of the lab50.
NOTE: The blood is collected into heparinized or EDTA-coated 0.5 mL tubes (see Table of Materials). - Maintain the collected blood samples at 4 °C until processed.
- To process the samples, first centrifuge at 208 x g for 10 min at 4 °C to separate the plasma.
- Determine the blood ethanol concentrations using an oxygen-rate ethanol analyzer (see Table of Materials) or other systems capable of determining BECs.
Representative Results
In the present representative study, ethanol intake (g/kg) during 2BC is reported during baseline drinking and after weeks of CIE (post vapor). Briefly, as described in the protocol, during 2BC, the mice had access to two bottles: one containing water and the other containing 15% (w/v) ethanol. After the baseline intakes were determined, the subjects were split and evenly assigned to the CIE or Air group. The initial baseline ethanol intake during the 3 week period stabilized at 2.00 g/kg ± 0.21 g/kg (n = 15) before the CIE (Figure 1A,B).
When looking at all the weeks of alcohol drinking, a two-way repeated measures ANOVA revealed a significant effect of CIE treatment, F(13,78) =7.471, p < 0.0001, and week, F(6,78) = 14.07, p < 0.0001, on ethanol intake, as well as a significant week treatment interaction effect, F(6,78) = 5.135, p = 0.0002, on ethanol intake. A Bonferroni post hoc test51 revealed that the ethanol-dependent (CIE) mice increased their ethanol intake significantly at post-vapor week (PV) 6 compared to their baseline drinking volume. The Bonferroni post hoc test further revealed that the dependent mice consumed significantly more ethanol than the non-dependent (Air) mice at PV6 (6.9772 g/kg ± 1.94 g/kg for CIE and 3.637 g/kg ± 0.52 g/kg for Air, p < 0.0001) (Figure 1A).
When looking at a direct comparison of baseline intake and the last week of alcohol drinking post-CIE (PV6), a two-way repeated measures ANOVA also revealed a significant effect of CIE treatment, F(1,13) = 3.858, p = 0.0713, a significant effect of week, F (1,13) = 25.77, p = 0.0002, and a significant week treatment interaction effect, F(1,13) = 5.716, p = 0.0326, on ethanol intake. The Bonferroni post hoc test also showed that at PV6, the ethanol-dependent mice had significantly higher ethanol intake compared to their own baseline and non-dependent mice (Figure 1B).
To assess the BECs during CIE, blood from the dependent mice was collected at the end of the CIE every week. An ideal range for the BECs of the mice during the CIE procedure is 150-250 mL/dL. A representative average weekly BEC (173.12 mg/dL ± 20.786 mg/dL) is shown in Figure 1C.
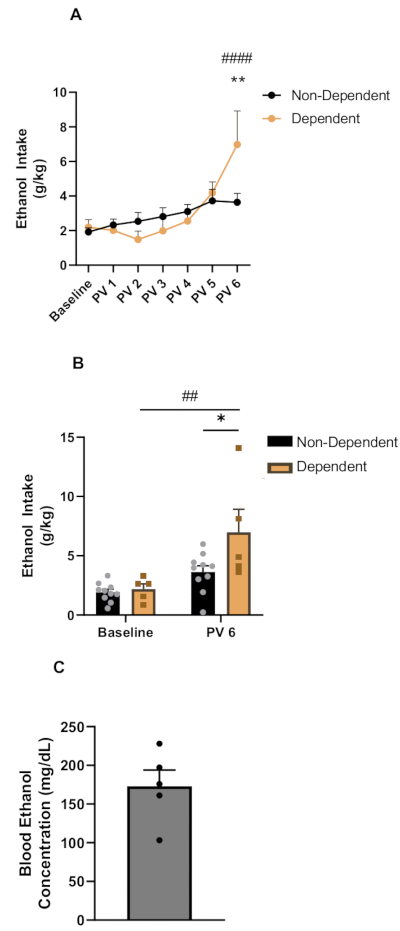
Figure 1: Ethanol intake during two-bottle choice drinking and blood ethanol concentrations post-CIE in male and female mice. (A) Two-bottle choice ethanol intake of the dependent (CIE; n = 5; gold circles) and non-dependent (Air; n = 10; black circles) mice over all the weeks. The ethanol intake of the CIE group was significantly higher than baseline levels after post-vapor week 6. The dependent mice also had higher alcohol intake compared to the Air exposure mice on post-vapor week 6. (B) Comparison of ethanol intake between baseline and post-vapor week 6. The dependent mice (gold bars) showed significantly higher ethanol intake than the non-dependent mice (black bars) on post-vapor week 6. The dependent mice also had significantly higher ethanol intake compared to their baseline intake during post-vapor week 6. (C) Representative blood ethanol concentrations for all the dependent mice. Data are shown as mean ± SEM. * = significant (p < 0.05) difference between non-dependent (black) and dependent group (gold) in PV6; ** p < 0.01; ## = significant (p = 0.001) difference between baseline and PV6 within the dependent group; #### p < 0.0001. Abbreviation: PV = post-vapor week. Please click here to view a larger version of this figure.
Supplementary Figure 1: Drinking bottles. Image of the hand-crafted drinking bottles for water (top bottle) and alcohol (bottom bottle). Each contains a rubber stopper on the end where the liquid is filled. f Please click here to download this File.
Supplementary Figure 2: Cage setup for the two-bottle choice procedure with ethanol and water. The bottle positions are rotated daily. (A) Top view. (B) Side view. Note that a clip is used to secure the ball-bearing sipper tube to the wire cage lid, and the sipper tube calibration markings on the bottles are oriented upward to enable the experimenter to record measures while the mice have access to ethanol. Please click here to download this File.
Supplementary Figure 3: Representation of the vapor chambers holding (A) mouse cages and (B) the overall ethanol inhalation system setup. Please click here to download this File.
Discussion
Alcohol use disorder represents a global public health problem with high prevalence and cost to society52. To study AUD in preclinical animal models, a common method in mice is 2BC/CIE20,34,39,40,47,53,54,55. Here, this established model of alcohol dependence was used to present representative methods and results from a standard 2BC/CIE study. As expected, this study showed that mice increased their intake of alcohol after several weeks of CIE compared to the Air controls and their own previous baseline alcohol intake. Following the protocol described in this manuscript, researchers can achieve sustained alcohol exposure (i.e., persistent blood and brain levels of alcohol) that leads to the development of withdrawal symptoms and, eventually, the escalation of alcohol intake. Animal withdrawal can manifest as increased irritability, aggression, sensory overreaction, abnormal activity, and central nervous system overexcitation33. Importantly, the withdrawal symptoms observed in animal models correspond to the symptoms of alcohol withdrawal in humans to a large extent3,4,33,56,57,58.
Excessive alcohol drinking can lead to alcohol dependence, a maladaptive neurophysiological state that leads to a range of withdrawal symptoms when alcohol consumption is drastically reduced or stopped altogether33,56,59. These symptoms often involve compensatory responses to the effects of alcohol, which are assumed to represent the brain's attempt to re-establish homeostasis during sustained exposure to alcohol58,60,61,62,63,64. Due to the limitations of human studies and the different aspects of alcohol withdrawal, clinical studies have had difficulty identifying the risk factors, vulnerability, and potential withdrawal mechanisms of human alcoholics65,66. Using animal models allows researchers to control and examine the neural, molecular, genetic, and environmental factors that promote alcohol dependence and withdrawal. The 2BC/CIE model has been used to induce alcohol dependence and withdrawal symptoms for the study of AUD. These withdrawal symptoms include hyperexcitability, irritability-like behavior, anxiety-like behavior, and sleep disorders3,14,22,28,44,57,67.
There are several significant advantages to the 2BC/CIE model. First, this is one of the only methods that results in the development of ethanol dependence and a significant escalation of ethanol intake. Although the CIE exposure is forced ethanol intake, there is a component of voluntary ethanol escalation through 2BC, which may be lacking in alternative models of AUD such as injection or oral gavage (i.e., quick models used to study the neural mechanisms of alcohol exposure/dependence)68,69,70. Ideally, mice can reach the target blood ethanol concentration level (150 mg%) within 1 week71. In addition, stable BECs can be maintained over a long period with normal weight regulation and feeding behavior71.
There are a few limitations to the current version of the 2BC/CIE method. Pyrazole has been widely used as an in vitro and in vivo ethanol dehydrogenase inhibitor. Since the metabolism of mice is faster than that of rats, the infusion of pyrazole before the CIE experiment starts can inhibit the function of alcohol dehydrogenase in mice, thus enhancing the level of intoxication in the mice72. However, pyrazole is highly toxic, and the long-term injection of pyrazole can result in weight loss, liver necrosis, and cytotoxicity in other organs, especially when administered with alcohol72. An inherent limitation of the 2BC method is that it is difficult to demonstrate the animal's motivation to obtain alcohol33. On the contrary, in operant models, the effort to achieve the alcohol can be measured using progressive ratio responding34,73,74. However, recent open-source models to design electronic 2BC equipment that counts the number of contacts with the sipper tube over time may help mitigate this issue54,75,76,77. Finally, one major caveat to the use of the 2BC/CIE model is that the development of alcohol dependence occurs through the forced chronic intake of alcohol vapor and not voluntary consumption, although the escalation of alcohol intake is expressed through voluntary 2BC consumption. Recently, differences in neural activity profiles in alcohol-dependent rats when using forced or voluntary vapor exposure methods have been identified74, which suggests that the lack of choice for the induction of dependence may lead to differences in neural responses.
In summary, while the alcohol field has various animal models to investigate the different physiological and behavioral aspects of AUD (for example, alcohol injection or gavage), the protocol described above is one of the most reliable methods for modeling AUD. Here, a protocol for the 2BC/CIE model of alcohol dependence in mice to study AUD is highlighted, which can be used for a variety of research questions, including the examination of the brain-wide neural circuits involved in AUD22,55,78,79. This method will continue to be ideal for the further examination of the neurobiological mechanisms of AUD80.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants AA027301 and AA029985.
Materials
| 500 Eppendorf Tubes | Eppendorf | L203896J | |
| 95% ethanol | Decon laboratories | 2816 | |
| Analox machine | Analox Instruments | Analox-AM1 | |
| Animal Weighing Scale | Kent Scientific | SCL-4000 | |
| Binder Clips | Office Depot | 560394 | |
| C57BL/6J mice | The Jackson Laboratory | 000664 | |
| Centrifuge | Eppendorf | 5418R | |
| Chronic intermitted vapor chamber | La Jolla Alcohol Research Inc | Custom made materials | |
| Heparin/EDTA | Sagent Pharmaceuticals | TS/DRUGS/2/2015 | |
| Mouse bedding | Bed-o’Cobs | 8B | fill with 1/8" deep |
| Mouse drinking bottle | Custom made materials | ||
| Pyrazole | Sigma-Aldrich | bccc6397 | |
| Teklad global 18% protein (mouse food) | Teklad global | Envigo 2018 |
References
- Koob, G. F., Volkow, N. D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 3 (8), 760-773 (2016).
- Witkiewitz, K., Litten, R. Z., Leggio, L. Advances in the science and treatment of alcohol use disorder. Science Advances. 5 (9), 4043 (2019).
- Becker, H. C. Alcohol dependence, withdrawal, and relapse. Alcohol Research and Health. 31 (4), 348-361 (2008).
- Becker, H. C. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research. 34 (4), 448-458 (2012).
- Erickson, E. K., Grantham, E. K., Warden, A. S., Harris, R. A. Neuroimmune signaling in alcohol use disorder. Pharmacology, Biochemistry, and Behavior. 177, 34-60 (2019).
- Aalsma, M., et al. Adolescent suicide assessment and management in primary care. BMC Pediatrics. 22 (1), 389 (2022).
- Grant, B. F., et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 72 (8), 757-766 (2015).
- Helle, A. C., Watts, A. L., Trull, T. J., Sher, K. J. Alcohol use disorder and antisocial and borderline personality disorders. Alcohol Research. 40 (1), (2019).
- Luderer, M., Ramos Quiroga, J. A., Faraone, S. V., Zhang James, Y., Reif, A. Alcohol use disorders and ADHD. Neuroscience and Biobehavioral Reviews. 128, 648-660 (2021).
- Ribadier, A., Varescon, I. Anxiety and depression in alcohol use disorder individuals: The role of personality and coping strategies. Substance Use and Misuse. 54 (9), 1475-1484 (2019).
- McHugh, R. K., Weiss, R. D. Alcohol use disorder and depressive disorders. Alcohol Research. 40 (1), (2019).
- Le Berre, A. P. Emotional processing and social cognition in alcohol use disorder. Neuropsychology. 33 (6), 808-821 (2019).
- Sontate, K. V., et al. Alcohol, aggression, and violence: From public health to neuroscience. Frontiers in Psychology. 12, 699726 (2021).
- Prescrire International. Alcohol withdrawal syndrome: how to predict, prevent, diagnose and treat it. Prescrire International. 16 (87), 24-31 (2007).
- Mendelson, J. H. The effect of Alcohol on the nervous system. Alcohol Health and Research World. 19 (1), 28-29 (1995).
- Murray, T. D., Berger, A. Alcohol withdrawal. Virginia Medical Quarterly. 124 (3), 184-187 (1997).
- Fama, R., et al. Memory impairment in alcohol use disorder is associated with regional frontal brain volumes. Drug and Alcohol Dependence. 228, 109058 (2021).
- Kopelman, M. D. The Korsakoff syndrome. British Journal of Psychiatry. 166 (2), 154-173 (1995).
- Griffin, W. C. Alcohol dependence and free-choice drinking in mice. Alcohol. 48 (3), 287-293 (2014).
- Griffin, W. C., Lopez, M. F., Yanke, A. B., Middaugh, L. D., Becker, H. C. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 201 (4), 569-580 (2009).
- Kimbrough, A., et al. CRF(1) receptor-dependent increases in irritability-like behavior during abstinence from chronic intermittent ethanol vapor exposure. Alcoholism: Clinical and Experimental Research. 41 (11), 1886-1895 (2017).
- Kimbrough, A., et al. Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proceedings of the National Academy of Sciences of the United States of America. 117 (4), 2149-2159 (2020).
- O’Dell, L. E., Roberts, A. J., Smith, R. T., Koob, G. F. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 28 (11), 1676-1682 (2004).
- Pleil, K. E., et al. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 99, 735-749 (2015).
- Warden, A. S., et al. Microglia control escalation of drinking in alcohol-dependent mice: Genomic and synaptic drivers. Biological Psychiatry. 88 (12), 910-921 (2020).
- Gilpin, N. W., Koob, G. F. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Research and Health. 31 (3), 185-195 (2008).
- Kreifeldt, M., et al. Central amygdala corticotropin-releasing factor neurons promote hyponeophagia but do not control alcohol drinking in mice. Molecular Psychiatry. 27 (5), 2502-2513 (2022).
- Sidhu, H., Kreifeldt, M., Contet, C. Affective disturbances during withdrawal from chronic intermittent ethanol inhalation in C57BL/6J and DBA/2J male mice. Alcoholism: Clinical and Experimental Research. 42 (7), 1281-1290 (2018).
- Kononoff, J., et al. Systemic and intra-habenular activation of the orphan G protein-coupled receptor GPR139 decreases compulsive-like alcohol drinking and hyperalgesia in alcohol-dependent rats. eNeuro. 5 (3), (2018).
- Avegno, E. M., et al. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. Journal of Neuroscience. 38 (36), 7761-7773 (2018).
- de Guglielmo, G., et al. Recruitment of a neuronal ensemble in the central nucleus of the amygdala is required for alcohol dependence. Journal of Neuroscience. 36 (36), 9446-9453 (2016).
- Liang, J., et al. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. Journal of Neuroscience. 26 (6), 1749-1758 (2006).
- Becker, H. C. Animal models of alcohol withdrawal. Alcohol Research and Health. 24 (2), 105-113 (2000).
- Kimbrough, A., Kim, S., Cole, M., Brennan, M., George, O. Intermittent access to ethanol drinking facilitates the transition to excessive drinking after chronic intermittent ethanol vapor exposure. Alcoholism: Clinical and Experimental Research. 41 (8), 1502-1509 (2017).
- Koob, G. F., Colrain, I. M. Alcohol use disorder and sleep disturbances: A feed-forward allostatic framework. Neuropsychopharmacology. 45 (1), 141-165 (2020).
- Risher, M. L., et al. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: Radial-arm maze performance and operant food reinforced responding. PLoS One. 8 (5), e62940 (2013).
- Van Skike, C. E., Novier, A., Diaz-Granados, J. L., Matthews, D. B. The effect of chronic intermittent ethanol exposure on spatial memory in adolescent rats: The dissociation of metabolic and cognitive tolerances. Brain Research. 1453, 34-39 (2012).
- Ho, A. M., et al. Chronic intermittent ethanol exposure alters behavioral flexibility in aged rats compared to adult rats and modifies protein and protein pathways related to Alzheimer’s disease. ACS Omega. 7 (50), 46260-46276 (2022).
- Piggott, V. M., Lloyd, S. C., Perrine, S. A., Conti, A. C. Chronic intermittent ethanol exposure increases ethanol consumption following traumatic stress exposure in mice. Frontiers in Behavioral Neuroscience. 14, 114 (2020).
- Quijano Carde, N. A., De Biasi, M. Behavioral characterization of withdrawal following chronic voluntary ethanol consumption via intermittent two-bottle choice points to different susceptibility categories. Alcoholism: Clinical and Experimental Research. 46 (4), 614-627 (2022).
- Rimondini, R., Arlinde, C., Sommer, W., Heilig, M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB Journal. 16 (1), 27-35 (2002).
- Becker, H. C. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 122, 115-126 (2017).
- Zhao, Y., Weiss, F., Zorrilla, E. P. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism: Clinical and Experimental Research. 31 (9), 1505-1515 (2007).
- Becker, H. C., Lopez, M. F. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 28 (12), 1829-1838 (2004).
- Kreifeldt, M., Le, D., Treistman, S. N., Koob, G. F., Contet, C. BK channel beta1 and beta4 auxiliary subunits exert opposite influences on escalated ethanol drinking in dependent mice. Frontiers in Integrative Neuroscience. 7, 105 (2013).
- Pringuey, D., et al. Comorbidity of affective disorders and alcohol use disorder. Encephale. 40, S3-S7 (2014).
- Okhuarobo, A., et al. A novel mouse model for vulnerability to alcohol dependence induced by early-life adversity. Neurobiology of Stress. 13, 100269 (2020).
- Dilley, J. E., Nicholson, E. R., Fischer, S. M., Zimmer, R., Froehlich, J. C. Alcohol drinking and blood alcohol concentration revisited. Alcoholism: Clinical and Experimental Research. 42 (2), 260-269 (2018).
- Feierman, D. E., Cederbaum, A. I. Oxidation of the alcohol dehydrogenase inhibitor pyrazole to 4-hydroxypyrazole by microsomes. Effect of cytochrome P-450 inducing agents. Drug Metabolism and Disposition. 15 (5), 634-639 (1987).
- Parasuraman, S., Raveendran, R., Kesavan, R. Blood sample collection in small laboratory animals. Journal of Pharmacology and Pharmacotherapeutics. 1 (2), 87-93 (2010).
- Armstrong, R. A. When to use the Bonferroni correction. Ophthalmic and Physiological Optics. 34 (5), 502-508 (2014).
- Rehm, J., et al. Alcohol use disorders in primary health care: What do we know and where do we go. Alcohol and Alcoholism. 51 (4), 422-427 (2016).
- Gorini, G., Roberts, A. J., Mayfield, R. D. Neurobiological signatures of alcohol dependence revealed by protein profiling. PLoS One. 8 (12), e82656 (2013).
- Frie, J. A., Khokhar, J. Y. An open source automated two-bottle choice test apparatus for rats. HardwareX. 5, 00061 (2019).
- Smith, R. J., et al. Dynamic c-Fos changes in mouse brain during acute and protracted withdrawal from chronic intermittent ethanol exposure and relapse drinking. Addiction Biology. 25 (6), e12804 (2020).
- Sachdeva, A., Choudhary, M., Chandra, M. Alcohol withdrawal syndrome: Benzodiazepines and beyond. Journal of Clinical and Diagnostic Research. 9 (9), (2015).
- Huitron-Resendiz, S., et al. Effects of withdrawal from chronic intermittent ethanol exposure on sleep characteristics of female and male mice. Alcoholism: Clinical and Experimental Research. 42 (3), 540-550 (2018).
- Gatch, M. B., Lal, H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcoholism: Clinical and Experimental Research. 23 (2), 328-333 (1999).
- Saitz, R. Introduction to alcohol withdrawal. Alcohol Health and Research World. 22 (1), 5-12 (1998).
- Emmerson, R. Y., Dustman, R. E., Shearer, D. E., Chamberlin, H. M. EEG, visually evoked and event related potentials in young abstinent alcoholics. Alcohol. 4 (4), 241-248 (1987).
- Gauvin, D. V., Youngblood, B. D., Holloway, F. A. The discriminative stimulus properties of acute ethanol withdrawal (hangover) in rats. Alcoholism: Clinical and Experimental Research. 16 (2), 336-341 (1992).
- George, O., Le Moal, M., Koob, G. F. Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiology and Behavior. 106 (1), 58-64 (2012).
- Koob, G. F., Le Moal, M. Addiction and the brain antireward system. Annual Review of Psychology. 59, 29-53 (2008).
- Koob, G. F., Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 24 (2), 97-129 (2001).
- Nieto, S. J., Grodin, E. N., Aguirre, C. G., Izquierdo, A., Ray, L. A. Translational opportunities in animal and human models to study alcohol use disorder. Translational Psychiatry. 11 (1), 496 (2021).
- Kuhns, L., Kroon, E., Lesscher, H., Mies, G., Cousijn, J. Age-related differences in the effect of chronic alcohol on cognition and the brain: A systematic review. Translational Psychiatry. 12 (1), 345 (2022).
- Becker, H. C., Mulholland, P. J. Neurochemical mechanisms of alcohol withdrawal. Handbook of Clinical Neurology. 125, 133-156 (2014).
- Chen, M. M., et al. Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation. Mediators of Inflammation. , 971481 (2013).
- Livy, D. J., Parnell, S. E., West, J. R. Blood ethanol concentration profiles: A comparison between rats and mice. Alcohol. 29 (3), 165-171 (2003).
- Jin, L., Batra, S., Jeyaseelan, S. Diminished neutrophil extracellular trap (NET) formation is a novel innate immune deficiency induced by acute ethanol exposure in polymicrobial sepsis, which can be rescued by CXCL1. PLoS Pathogens. 13 (9), e1006637 (2017).
- Crabbe, J. C., Harris, R. A., Koob, G. F. Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences. 1216, 24-40 (2011).
- Blomstrand, R., et al. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: An approach to therapy against methanol poisoning. Proceedings of the National Academy of Sciences of the United States of America. 76 (7), 3499-3503 (1979).
- Domi, A., Stopponi, S., Domi, E., Ciccocioppo, R., Cannella, N. Sub-dimensions of alcohol use disorder in alcohol preferring and non-preferring rats, a comparative study. Frontiers in Behavioral Neuroscience. 13, 3 (2019).
- de Guglielmo, G., et al. Voluntary and forced exposure to ethanol vapor produces similar escalation of alcohol drinking but differential recruitment of brain regions related to stress, habit, and reward in male rats. Neuropharmacology. 222, 109309 (2023).
- Haggerty, D. L., et al. The role of anterior insular cortex inputs to dorsolateral striatum in binge alcohol drinking. Elife. 11, 77411 (2022).
- Slivicki, R. A., et al. Oral oxycodone self-administration leads to features of opioid misuse in male and female mice. Addiction Biology. 28 (1), e13253 (2023).
- Godynyuk, E., Bluitt, M. N., Tooley, J. R., Kravitz, A. V., Creed, M. C. An open-source, automated home-cage sipper device for monitoring liquid ingestive behavior in rodents. eNeuro. 6 (5), (2019).
- Simpson, S., et al. The hidden brain: Uncovering previously overlooked brain regions by employing novel preclinical unbiased network approaches. Frontiers in Systems Neuroscience. 15, 595507 (2021).
- Smith, L. C., Kimbrough, A. Leveraging neural networks in preclinical alcohol research. Brain Sciences. 10 (9), 578 (2020).
- Hwa, L., Besheer, J., Kash, T. Glutamate plasticity woven through the progression to alcohol use disorder: A multi-circuit perspective. F1000Research. 6, 298 (2017).

.
