Tracking Electrochemistry on Single Nanoparticles with Surface-Enhanced Raman Scattering Spectroscopy and Microscopy
Summary
The protocol describes how to monitor electrochemical events on single nanoparticles using surface-enhanced Raman scattering spectroscopy and imaging.
Abstract
Studying electrochemical reactions on single nanoparticles is important to understand the heterogeneous performance of individual nanoparticles. This nanoscale heterogeneity remains hidden during the ensemble-averaged characterization of nanoparticles. Electrochemical techniques have been developed to measure currents from single nanoparticles but do not provide information about the structure and identity of the molecules that undergo reactions at the electrode surface. Optical techniques such as surface-enhanced Raman scattering (SERS) microscopy and spectroscopy can detect electrochemical events on individual nanoparticles while simultaneously providing information on the vibrational modes of electrode surface species. In this paper, a protocol to track the electrochemical oxidation-reduction of Nile Blue (NB) on single Ag nanoparticles using SERS microscopy and spectroscopy is demonstrated. First, a detailed protocol for fabricating Ag nanoparticles on a smooth and semi-transparent Ag film is described. A dipolar plasmon mode aligned along the optical axis is formed between a single Ag nanoparticle and Ag film. The SERS emission from NB fixed between the nanoparticle and the film is coupled into the plasmon mode, and the high-angle emission is collected by a microscope objective to form a donut-shaped emission pattern. These donut-shaped SERS emission patterns allow for the unambiguous identification of single nanoparticles on the substrate, from which the SERS spectra can be collected. In this work, a method for employing the SERS substrate as a working electrode in an electrochemical cell compatible with an inverted optical microscope is provided. Finally, tracking the electrochemical oxidation-reduction of NB molecules on an individual Ag nanoparticle is shown. The setup and the protocol described here can be modified to study various electrochemical reactions on individual nanoparticles.
Introduction
Electrochemistry is an important measurement science for studying charge transfer, charge storage, mass transport, etc., with applications in diverse disciplines, including biology, chemistry, physics, and engineering1,2,3,4,5,6,7. Conventionally, electrochemistry involves measurements over an ensemble — a large collection of single entities such as molecules, crystalline domains, nanoparticles, and surface sites. However, understanding how such single entities contribute to ensemble-averaged responses is key for bringing forth new fundamental and mechanistic understandings in chemistry and related fields because of the heterogeneity of electrode surfaces in complex electrochemical environments8,9. For example, ensemble reduction has revealed site-specific reduction/oxidation potentials10, the formation of intermediates and minor catalysis products11, site-specific reaction kinetics12,13, and charge carrier dynamics14,15. Reducing ensemble averaging is particularly important in improving our understanding beyond model systems to applied systems, such as biological cells, electrocatalysis, and batteries, in which extensive heterogeneity is often found16,17,18,19,20,21,22.
In the past decade or so, there has been an emergence of techniques to study single-entity electrochemistry1,2,9,10,11,12. These electrochemical measurements have provided the capabilities to measure small electrical and ionic currents in several systems and revealed new fundamental chemical and physical characteristics23,24,25,26,27,28. However, electrochemical measurements do not provide information about the identity or structure of molecules or intermediates at the electrode surface29,30,31,32. Chemical information at the electrode-electrolyte interface is central to understanding electrochemical reactions. Interfacial chemical knowledge is typically obtained by coupling electrochemistry with spectroscopy31,32. Vibrational spectroscopy, such as Raman scattering, is well-suited to provide complementary chemical information on charge transfer and related events in electrochemical systems that predominately utilize, but are not limited to, aqueous solvents30. Coupled with microscopy, Raman scattering spectroscopy provides spatial resolution down to the diffraction limit of light33,34. Diffraction presents a limitation, however, because nanoparticles and active surface sites are smaller in length than optical diffraction limits, which, thus, precludes the study of individual entities35.
Surface-enhanced Raman scattering (SERS) has been demonstrated to be a powerful tool in studying interfacial chemistry in electrochemical reactions20,30,36,37,38. In addition to providing the vibrational modes of reactant molecules, solvent molecules, additives and the surface chemistries of electrodes, SERS provides a signal that is localized to the surface of materials that support collective surface electron oscillations, known as localized surface plasmon resonances. The excitation of plasmon resonances leads to the concentration of electromagnetic radiation at the surface of the metal, thus increasing both the flux of light to and the Raman scattering from surface adsorbates. Nanostructured noble metals such as Ag and Au are commonly used plasmonic materials because they support visible light plasmon resonances, which are desirable for detecting emission with highly sensitive and efficient charge-coupled devices. Although the largest enhancements in SERS come from aggregates of nanoparticles39,40, a new SERS substrate has been developed that allows SERS measurements from individual nanoparticles: gap-mode SERS substrate (Figure 1)41,42. In gap-mode SERS substrates, a metallic mirror is fabricated and coated with an analyte. Next, nanoparticles are dispersed over the substrate. When irradiated with circularly polarized laser light, a dipolar plasmon resonance formed by the coupling of the nanoparticle and substrate is excited, which enables SERS measurements on single nanoparticles. SERS emission is coupled to the dipolar plasmon resonance43,44,45, which is oriented along the optical axis. With the parallel alignment of the radiating electric dipole and collection optics, only high-angle emission is collected, thus forming distinct donut-shaped emission patterns46,47,48,49 and allowing the identification of single nanoparticles. Aggregates of nanoparticles on the substrate contain radiating dipoles that are not parallel to the optical axis50. In this latter case, low-angle and high-angle emissions are collected and form solid emission patterns46.
Here, we describe a protocol for fabricating gap-mode SERS substrates and a procedure to employ them as working electrodes to monitor electrochemical redox events on single Ag nanoparticles using SERS. Importantly, the protocol using gap-mode SERS substrates allows for the unambiguous identification of single nanoparticles by SERS imaging, which is a key challenge for current methodologies in single nanoparticle electrochemistry. As a model system, we demonstrate the use of SERS to provide a readout of the electrochemical reduction and oxidation of Nile Blue A (NB) on a single Ag nanoparticle driven by a scanning or stepped potential (i.e., cyclic voltammetry, chronoamperometry). NB undergoes a multi-proton, multi-electron reduction/oxidation reaction in which its electronic structure is modulated out of/in resonance with the excitation source, which provides a contrast in the corresponding SERS spectra10,51,52. The protocol described here is also applicable to non-resonant redox-active molecules and electrochemical techniques, which may be pertinent to applications such as electrocatalysis.
Protocol
1. Gap-mode SERS substrate preparation
- Clean No. 1 coverslips (see Table of Materials) using an acetone and water wash, as described below. Perform this step in a cleanroom to ensure that no debris or other unwanted matter is deposited onto the coverslips.
- Place the coverslips in a slide rack. Use tweezers when moving the coverslips/substrates. Place the slide rack in a glass container, and fill it with acetone.
CAUTION: Acetone is highly flammable and has potential negative health effects. Handle it in a well-ventilated area using gloves, goggles, and a mask. - Adjust the power control of the ultrasonic generator to 8, and sonicate the glass container with the slide rack for 15 min.
- Remove the slide rack from the container, and rinse the slide rack and coverslips thoroughly with ultrapure (resistivity of 18.2 MΩ·cm) water.
- Place the slide rack with coverslips in a glass container, and fill it with ultrapure water. Sonicate the glass container with the slide rack for another 15 min using the same settings.
- Remove the slide rack from the container, and wash the slide rack and coverslips thoroughly with ultrapure water.
- Using a spray gun, dry the coverslips with a stream of high-purity N2 gas.
- Place the coverslips in a slide rack. Use tweezers when moving the coverslips/substrates. Place the slide rack in a glass container, and fill it with acetone.
- Deposit Cu and Ag on the cleaned coverslips. To do this, use the electron beam thin film deposition system following standard procedures, as recommended by the manufacturer in the official user manual.
NOTE: For any other deposition, please follow the instructions given by the manufacturer, as provided at the institutional facilities53.- Set the platen position to 180°, and vent the vacuum chamber.
- Arrange the clean coverslips side by side in the instrument's platen so that they do not overlap. Use heat-resistant adhesive tape (polyimide film) to attach the coverslips to the platen.
NOTE: This ensures that the coverslips do not move or fall during the procedure. - Fill a graphite crucible halfway with Cu pellets, and insert it into the crucible holder. Do the same for Ag in a second crucible. Close the vacuum chamber, and start pumping down; the recommended deposition pressure is in the order of 10−7 – 10−6 Torr.
- Load the Cu properties into the sensor's application. Turn on the platen rotation at 20 RPM. Set the platen position to 225°.
NOTE: This positions the mirror on the bottom of the platen in a way that the electron beam can be seen from the view port. - Turn on the breaker to the electron beam power supply, and wait at least 2 min. Turn on the electron beam, and wait another 2 min. Open the substrate shutter.
NOTE: This makes the beam and crucible visible through the mirror. - Gradually (at around 10 mA/min) increase the emission current until the sensor reads a deposition rate close to 10 Å/s. Close the shutter, and set the platen position to 0°.
NOTE: The beam may change shape during this process. It is important to check it regularly during this step and correct its position, amplitude, and frequency using the respective knobs. The beam must be heating the contents of the crucible evenly. Closing the shutter at this point ensures that no metal is deposited onto the samples as the platen rotates to position the coverslips in the path of the evaporated metal. - Open the shutter to start the deposition, and monitor the thickness as displayed by the sensor. Close the shutter when the desired thickness is reached (1 nm for Cu), as determined by the deposition sensor.
- Gradually decrease the current of the electron beam until the sensor reads close to 0 A but the current is high enough that the crucible is visible.
- Set the position of the platen to 225°, and open the shutter to be able to see the crucible.
- Rotate the crucible holder using the knob so that the beam is directed toward the crucible with Ag pellets.
- Load the Ag properties into the sensor's application. Repeat steps 1.2.6 – 1.2.7, but use a deposition rate of 20 Å/s and a thickness of 25 nm for Ag.
- Gradually decrease the current to 0 A, and turn off the electron beam and the breaker. Set the position of the platen to 180°, and vent the vacuum chamber. Open the vacuum chamber.
- The coverslips should be in the same place as before, free of foreign matter or dust particles, and with the appearance of a mirror. Slowly and carefully remove the heat-resistant adhesive tape.
NOTE: Pull the tape back, parallel to the surface of the platen; there is a risk of breaking the coverslips. The film should be homogeneous and partially transparent (see Figure 2A).
- Incubate the Ag thin film with a Nile Blue solution, as described below.
- Add 500 µL of 50 µM NB solution onto the surface of the Ag thin film.
- After 15 min, rinse the Ag thin film thoroughly with ultrapure water to remove any weakly adsorbed NB molecules. Dry the Ag thin film with N2 gas.
- Drop-cast Ag nanoparticles onto the NB-incubated Ag thin film. Add 500 µL of a 100x dilution of the Ag nanoparticle colloid onto the same region of the Ag thin film where the NB solution was drop-casted and incubated.
CAUTION: Metal nanoparticles are toxic to the human body. Handle them in a well-ventilated area using gloves and goggles. - After 20 min, rinse the (gap-mode SERS) substrate with ultrapure water. Dry the substrate with N2 gas.
2. Gap-mode SERS substrate characterization
- Ultraviolet-visible spectroscopy
- Turn on the instrument by pressing the power button. Launch the Scan software by double-clicking on its shortcut on the desktop.
- Click on Setup to open the setup window. Under Y Mode, click on the Mode drop-down menu, and select %T to measure the transmittance. Under X Mode, change Start to 800 and Stop to 200 in order to scan from 800 nm to 200 nm.
- In the Baseline tab, select the Baseline correction radio button, and close the setup window. Click on Baseline to perform a background correction with atmospheric air.
- Open the sample compartment. Tape one end of the Ag film onto the sample holder, perpendicular to the path of the beam.
- Click on Start to obtain a transmittance spectrum from the sample.
- Atomic force microscopy (AFM) measurements
- Connect the AFM to the computer (using a USB port), power on the AFM instrument, and launch Nanosurf Easyscan 2.
- Gently remove the AFM head (which has the AFM cantilever on the underside) from the sample stage, and place it aside upside down.
- Fix an Ag thin film substrate onto the sample stage using tape. Place the AFM head above the sample stage. Make sure the AFM head is parallel to the sample stage (monitor with the level indicator). If the AFM head and sample stage are not level, use the leveling screws to adjust the stage and to center the leveling bubble inside the level indicator.
- Using the side and top views in the software, gently move the sample stage as close to the AFM head (AFM cantilever) as possible without making contact. Make sure that the sample stage is not touching the AFM cantilever on the AFM head.
- Under the Acquisition tab, choose Phase Contrast as the imaging mode and PPP-XYNCHR as the cantilever type. Click on Laser Align to ensure that the laser is focused on the cantilever tip and the beam reflected from the tip is striking the center of the photodiode detector.
- Measure the resonant vibrational frequency of the cantilever with the AFM software by clicking on the Frequency Sweep button, and make sure that the frequency curve has a bell shape. By clicking on Approach, land the cantilever tip on the Ag thin film's surface.
- In the Imaging Wizard, choose an Imaging Size of 10.8 µm x 10.8 µm and a scan speed of 0.5 s/line. Under Z-controller, use a Setpoint of 50%, a P-Gain of 2,500, and an I-Gain of 2,500. Under Mode Properties, use a Free Vibration Amplitude of 300 mV.
- Click on Start to acquire an image. Save the image by right-clicking on it, select Copy, and paste it in the image processor.
- On the AFM software, choose the image to be processed by clicking on it. Under the Analysis tab, perform an area and line roughness analysis by clicking on Calculate Line Roughness and Calculate Area, respectively.
- Withdraw the cantilever tip from the Ag thin film surface by clicking on Withdraw. Move the sample stage away from the tip by monitoring the movement using the side and top views. Remove the sample.
- Scanning electron microscopy (SEM) measurements54
- Drop-cast 30 µL of the as-received Ag nanoparticle colloid onto a Si wafer, and let it air-dry completely. Fix the Si wafer on a sample stub using double-sided conductive tape.
- Vent the SEM chamber using the instrument's user interface. Slide open the SEM chamber, and mount the stub on one of the holes in the stage.
- Close the SEM chamber, and pump down the SEM chamber using the instrument's user interface.
- Position the sample approximately 10 mm away from the electron beam gun. Turn on the electron beam using the instrument's user interface.
- Image the sample using an Everhart-Thornley detector with a spot size of 6, a beam current of 25 pA, and a high voltage of 5 kV.
- Double-click on the area of interest for the electron gun to automatically align the electron beam. Perform imaging at a magnification of 3,500x using the instrument's user interface (Figure 3A).
- After the imaging is complete, turn off the electron beam, and move the sample away from the electron beam gun at least 20 mm.
- Vent the SEM chamber. Slide open the SEM chamber, and remove the sample stub from the stage. Close the SEM chamber, and pump it down using the instrument's user interface.
3. Preparation of the electrochemical cell
- Obtain a 5 cm long glass well by cutting a glass tube with a glass tube cutter, as described below.
- Wrap the chains of the glass tube cutter around the tube. Attach the last segment of the chain to the other side of the tool.
- Using one hand, hold the tool by the handle. With the other hand, hold the glass tube. Rotate the glass tube continuously so that the wheels in the chain start cutting the glass.
- Gently squeeze the tool by gradually applying more force on the handles. When the sound changes from sliding to scratching, that is when the glass piece (well) is about to separate from the glass tube.
- Smoothen the broken end of the glass well with 120-grit (or coarser) sandpaper. Polish with 220-grit (or finer) sandpaper.
- Cut down the gap-mode substrate with a diamond scribe, as described below.
- Place the gap-mode substrate on a flat surface. Move the diamond scribe up and down in the middle of the gap-mode substrate while applying light pressure on the surface of the substrate.
- Break the substrate into two pieces manually once a scratch is visible.
- Attach the cut glass well (from step 3.1) to the surface of the substrate, as described below.
- Dispense two-part epoxy resin onto a small sheet of aluminum foil. Mix the product using a stir stick or a pipette tip.
- Apply the mixture to the bottom rim of the glass well. Apply the minimum possible mixture to cover the rim of the cut glass well to minimize the spread of the resin to the inner part of the cell.
- Glue the glass well to the surface of the gap-mode substrate. Apply the remaining mixed product on the outsides of the well, where it meets the substrate, to eliminate the chance of leakage of the solution poured inside the glass well (see Figure 4A).
- Let the epoxy cure undisturbed for 5 min.
- Attach the electrical connection to the gap-mode SERS substrate, as described below.
- Obtain a 5 cm long copper wire. Dispense two-part conductive epoxy resin onto a small sheet of aluminum foil. Mix the product components using the copper wire.
- Attach the wire onto the surface of the substrate (outside the well, but attached to the conductive Ag thin film; see Figure 4A). Let the conductive epoxy cure undisturbed for the recommended time.
NOTE: It is recommended to let the conductive epoxy cure at room temperature to minimize the thermal annealing of the Ag film substrate.
4. Bulk cyclic voltammetry measurements
- Add 10 mL of 0.5 mM NB and 0.1 M phosphate buffer (pH = 5) to a 20 mL beaker. Insert a mechanically polished Ag disk electrode, a Pt wire, and an Ag/AgCl (3 M KCl) electrode into the electrolyte solution.
- Attach each electrode to its respective potentiostat clip (determined by the potentiostat's manufacturer). Make sure that the electrodes are not in contact with each other.
- Perform cyclic voltammetry (CV) from 0 to −0.6 V with a scan rate of 50 mV/s.
5. Single-nanoparticle electrochemical SERS microscopy and spectroscopy measurements
- Place the electrochemical cell prepared using the gap-mode SERS substrate on the stage of an inverted optical microscope.
- Tape the edges of the substrate onto the microscope stage so that it does not move during the spectroelectrochemical measurements due to the tension of the wires connecting the cell to the potentiostat (see Figure 4B).
- Place the Ag/AgCl (3 M KCl) reference electrode into the home-built stand, and fix its position by tightening the screw on the electrode holder stand.
- Clip the reference electrode to the potentiostat's reference electrode alligator clip (white color). Clip the Pt wire counter electrode to the potentiostat's counter electrode alligator clip (red color). Clip the Cu wire attached to the Ag film to the potentiostat's working electrode alligator clip (green color).
- Insert the Pt wire along with the alligator clip into the electrode holder, and tighten the screw to fix its position.
- Place the electrode holder over the electrochemical cell to insert the electrodes into the cell. Be careful not to let the electrodes touch the Ag film; not only will this form a short circuit, but it will also damage the film.
- Turn on the spectrometer and the EMCCD camera, and launch the "LightField" software.
- Turn on the 642 nm laser, and adjust the laser to a power of 500 µW.
CAUTION: Exposure to laser light may cause permanent damage to the eyes and skin. Consult and follow the safety guidelines of the relevant official regulatory body in your country/region. - Add a drop of immersion oil onto the objective. Move the focus knob to carefully raise the objective until the oil contacts the bottom of the substrate.
NOTE: Since the cell is taped down, forcing the objective up against the substrate may break the cell and/or damage the objective. - Focus the laser onto the surface of the gap-mode SERS substrate. Scan the gap-mode SERS substrate (covered by the glass well) to search for an isolated donut-shaped NB SERS emission pattern by moving the microscope stage (see Figure 5A).
NOTE: The lower the concentration of the NB, the harder it becomes to find donut-shaped emissions patterns but the higher the likelihood of the eventual donut-shaped emission pattern being isolated. Coffee rings are a good place to start, and then one can move inward with respect to the NB and Ag nanoparticle incubation area on the gap-mode SERS substrate. Cameras (see next step) are helpful in this process since they are more sensitive to light than the human eye when scanning around the gap-mode SERS substrate. - Attach a phone to the microscope phone adapter. To align the phone's camera with the adapter's lens, turn on the camera application on the phone, and change the position of the device to see through the lens.
- Remove one of the microscope's eyepieces, and insert the adapter in its place. On the camera application, change the mode to video, and zoom in as much as possible. The donut-shaped emission pattern can be seen clearly.
- Once the donut-shaped emission pattern is clearly located, move the light diverter lever of the microscope to direct the emitted light to the spectrometer.
- In the Experiment tab of LightField, click on Common Acquisition Settings, and adjust the Exposure Time to 0.1 s and the Frames to Save to 50. Under Export Data, select Export Acquired Data, and change the File Type to CSV (.csv).
- Under Regions of Interest, select the Custom Regions of Interest radio button. Click on Edit ROIs, and, in the new window, create a 25 pixel x 25 pixel ROI around the donut-shaped emission by changing the X, Y, W, and H values.
- Under Spectrometer, select the 600 g/mm, 750 nm blaze grating. Change the Center Wavelength to 642 nm. Click on Acquire to start the measurements.
- After the acquisition is finished, go to the Data tab. Open the last performed experiment, and click on Processes and then Frame combination.
- In the combined spectrum, take a note of the laser wavelength at which the highest intensity is observed.
- Go back to Experiment, and under Spectrometer, click on nm. In the popup window, change the measurement mode to relative wavenumbers, and enter the measured laser wavelength in the box. Change the grating position to 1,000/cm in order to detect Stokes-shifted Raman scattering from around 400/cm to 1,600/cm.
- Collect and sum at least 50 frames of NB SERS spectra using a 0.1 s exposure time (see Figure 5C). Look for a strong peak at 592/cm to confirm the emission is from NB (see Figure 5C)52. Take a SERS spectrum of the region adjacent to the donut-shaped emission pattern (a region with no emission) to compensate for the background signal.
- Keeping the laser light focused on the donut-shaped emission pattern, add 3 mL of the 0.1 M phosphate buffer solution (pH = 5) into the electrochemical cell using a 5 mL adjustable pipette.
NOTE: Once the electrolyte solution is added, the donut-shaped emission pattern may disappear, and a solid emission pattern may appear, as the dipole modes of the single nanoparticle off the optical axis radiate SERS spectra of the electrolyte and solvent molecules. - Refocus, if necessary, and make sure the laser light is still focused on the emission pattern.
- In the potentiostat's software, prepare a cyclic voltammogram experiment with at least three cycles from 0 to −0.6 V versus Ag/AgCl (3 M KCl) and a scan rate of 50 mV/s. For synchronizing the spectral and electrochemical data collection, configure the potentiostat to be triggered by the spectral acquisition of the spectrometer.
- Run the simultaneous CV and SERS experiments. The NB SERS spectra should be modulated by the potential applied to the gap-mode SERS substrate (see Figure 6B).
- Move the light diverter lever so that the light is directed to the phone camera. Start recording a video, and run the CV experiment as described. The SERS image intensity should be modulated as per the potential applied to the gap-mode SERS substrate (see the insets in Figure 6A).
6. Imaging analysis
- Process the collected images to enhance the sharpness and contrast, as described below.
NOTE: Image processing was performed with the OpenCV library in Python, and the script is available on GitHub (github.com/jvhemmer/jove_specsers).- Crop the image to remove most of the blank space, and center it around the emission pattern.
- Delete the green and blue channels of the frame. Increase the sharpness by subtracting a Gaussian-blurred mask of the frame.
- Increase the contrast by dynamic range expansion with a raise-to-power operator.
- Add scale bars onto the images using ImageJ, as described below.
- Using the phone camera adapter, image an object with known dimensions, such as a microscope calibration slide.
- Using ImageJ, load the collected image. Draw a segment on an area of the imaged object with known dimensions.
- Set the scale (i.e., pixels per unit distance) based on the length of the drawn segment using the Set Scale function. Add the scale using the Scale tool.
7. Nanoparticle size analysis
- Load the SEM image into ImageJ. Draw a segment on the instrument-provided scale bar, and set that using the Set Scale function.
- Go to Image > Type > 16-bit. Go to Image > Adjust > Auto threshold. From the drop-down menu, select Default.
- Using the Rectangle tool, select and delete the features that are not single nanoparticles.
- Use the Analyze Particles tool. Calculate the diameter of the particles with the obtained areas by assuming a circular shape.
8. Spectroelectrochemical data analysis
- Perform background correction on the collected spectral data. Perform data processing and plotting in MATLAB; the scripts are available on the same GitHub repository as previously mentioned.
- Average the spectral data from three different background experiments (spectral data collected alongside the SERS emission pattern). Subtract the average background spectrum from the sample's spectrum.
- Create a time array from 0 to the total time of the experiment (cyclic voltammetry), in which the interval is the sum of the exposure time, EMCCD readout time, and shutter open and close times.
- Convert the wavelength measurements to Raman shift using the laser wavelength.
- Generate a waterfall plot using the mesh function of MATLAB, where X is the Raman shift, Y is time, and Z is the intensity.
Representative Results
Figure 2A shows Ag thin film substrates prepared using an electron beam metal deposition system. The "good" substrate shown in Figure 2A has a homogenous coverage of Ag metal over the glass coverslip, while the "bad" substrate has a non-uniform coverage of Ag. The ultraviolet-visible spectrum of the "good" Ag thin film is shown in Figure 2B, which demonstrates that the film is partially transparent for the visible portion of the electromagnetic spectrum. The "good" Ag thin film substrate has an optical transparency of 34% for the 642 nm laser light that is used for the spectroelectrochemistry experiments in the current protocol. Figure 2C shows a representative AFM image of a 10.8 µm x 10.8 µm area of the "good" substrate. The root mean square roughness value of the representative area is 0.7 nm, which indicates that the Ag thin film is atomically smooth. The variation in the height of the Ag thin film substrate is represented by the line profile shown in Figure 2D, further demonstrating the uniformity and smoothness of the film.
Figure 3A shows a representative SEM image of Ag nanoparticles drop-cast and air-dried on a Si wafer. From an analysis of 243 nanoparticles, the average diameter of the Ag nanoparticles used in this protocol was 79.2 nm ± 8.4 nm. It should be noted that different sizes of Au or Ag nanoparticles could also be used55. Additionally, this protocol uses highly monodisperse nanoparticles, but there is no dispersity requirement, as this protocol enables the measurement of single nanoparticles. To construct a gap-mode SERS substrate, in this work, the Ag nanoparticles were deposited onto the surface of an Ag thin film substrate that had been previously incubated with NB (Figure 3B).
A gap-mode SERS substrate was used as the working electrode to construct an electrochemical cell, as shown in Figure 4A. The electrochemical cell was immobilized on a microscope stage and connected to a potentiostat, as shown in Figure 4B. With the electrochemical cell mounted on an inverted optical microscope, a 642 nm laser was focused onto the gap-mode SERS substrate working electrode in an epi-illumination geometry. Individual Ag nanoparticles on the Ag thin film in air can be unambiguously identified by a donut-shaped emission pattern, as shown in Figure 5A. These donut-shaped emission patterns can be reliably used as a signature to identify individual Ag nanoparticles49. If more than a single nanoparticle (dimer, trimer, or multimer) is present in the illumination volume, a solid emission pattern is observed, as shown in Figure 5B. Upon the introduction of the electrolyte solution, the donut-shaped emission pattern typically is converted to a solid emission pattern. The reason for this is that the dipolar plasmon modes within the single nanoparticle (not aligned with the optical axis) radiate emission from the solvent and electrolyte molecules in all directions. Therefore, the emission pattern is a superposition of high-angle NB SERS emission from the nanoparticle-substrate gap and low-angle SERS emission from the electrolyte and solvent molecules. The removal of the electrolytic solution recovers the donut-shaped emission patterns. In this protocol, following the identification of a single nanoparticle by SERS imaging, SERS spectroscopy is used to identify the redox probe molecule. The SERS spectrum in Figure 5C corresponds to the donut-shaped emission pattern shown in Figure 5A. The vibrational modes represent a fingerprint for the NB molecules.
Figure 6A displays representative cyclic voltammograms of NB in phosphate buffer (pH = 5) obtained using an Ag disk working electrode and a Pt wire counter electrode. A cyclic voltammogram is obtained prior to spectroelectrochemistry measurements of single nanoparticles to understand the ensemble redox behavior of the probe molecules — NB in this case. In this work, as the applied potential was swept from 0 to −0.6 V, a cathodic peak was observed at −0.27 V versus Ag/AgCl (3 M KCl). As the potential was swept back to 0 V, an anodic peak was observed at −0.21 V. The same applied potential range was used for the spectroelectrochemical measurements, as shown in Figure 6B. After the identification of a single Ag nanoparticle exhibiting a donut-shaped emission pattern, the electrolyte solution was pipetted into the electrochemical cell. Under laser illumination, the SERS spectra were then continuously collected as the applied potential was swept between 0 to −0.6 V at a scan rate of 50 mV/s (Figure 6B). The NB molecules in and around the gap between the Ag nanoparticle and the Ag film were electrochemically reduced (off state), and the SERS intensity decreased, as shown in the waterfall plot of the SERS spectra (also an inset in Figure 6A). As the applied potential was swept from −0.6 to 0 V, the SERS intensity increased, as the NB molecules were electrochemically oxidized (on state). The modulation in SERS signals represents a method to determine the reduction and oxidation potentials of NB on a single nanoparticle. Other electrochemical techniques can be substituted for voltammetry to further characterize redox reactions. Figure 7A shows the SERS response from NB when the potential of the working electrode was stepped to −0.4 V (i.e., chronoamperometry). When the electrode potential was stepped to −0.4 V, the SERS signal decayed due to the reduction of NB. This spectroelectrochemical technique enables one to investigate the transient behavior of redox reactions at the single-nanoparticle level. Figure 7B demonstrates how the reduction kinetics were altered by the magnitude of the electric bias applied, as evidenced by the decay of the area under the 592/cm peak. Interestingly, the sharp variations in the normalized area demonstrate how stochastic events play a larger role at this scale. As demonstrated with conventional voltammetry and chronoamperometry, the protocol described in this article allows researchers to track the vibrational modes of molecules as they are electrochemically reduced or oxidized on a single nanoparticle. Further, vibrational analyses of molecules on the surface of single nanoparticles allow for the differentiation between chemical and electrochemical steps, which is useful in studying reaction mechanisms.
Figure 1: Gap-mode SERS substrate. Schematic of a gap-mode substrate prepared by placing individual metal nanoparticles on a metal mirror. Please click here to view a larger version of this figure.
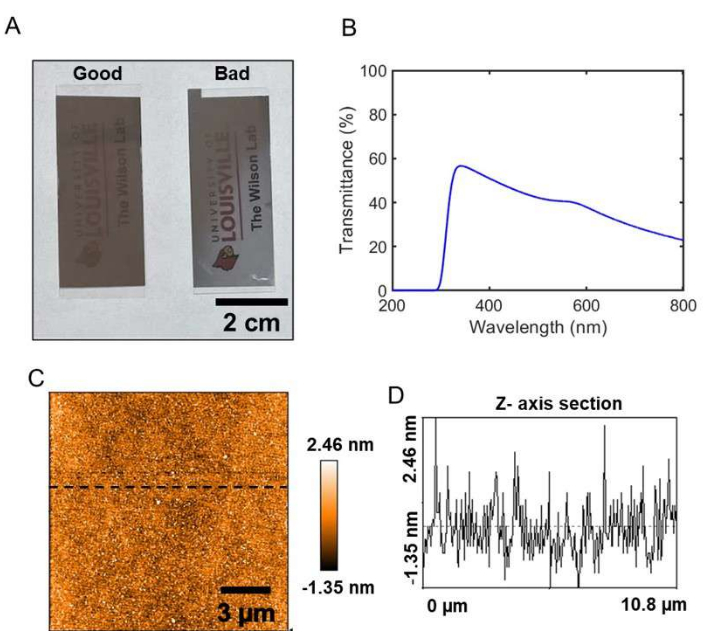
Figure 2: Ag thin film substrate characterization. (A) Digital photographs of a good and a bad Ag thin film substrate prepared by an electron beam metal evaporation system. (B) An ultraviolet-visible transmittance spectrum of a good substrate. (C) An AFM image of a representative 10.8 µm x 10.8 µm area of a good substrate. (D) A line profile of the AFM image indicated by the black dashed line shown in (C). Please click here to view a larger version of this figure.
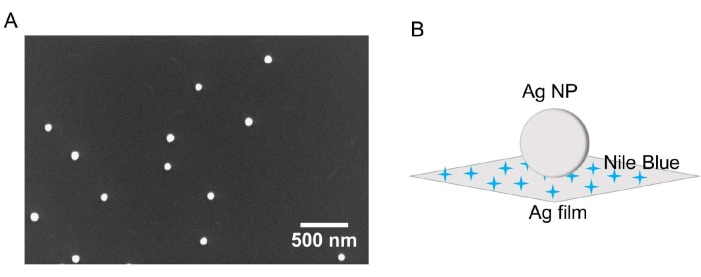
Figure 3: Ag nanoparticle characterization. (A) SEM image of an aqueous Ag nanoparticle colloid drop cast and air dried on a Si wafer. The average diameter of the nanoparticles is 79.2 nm, with a standard deviation of 8.4 nm. (B) Schematic diagram of the gap-mode SERS substrate. The blue stars represent NB molecules. Please click here to view a larger version of this figure.
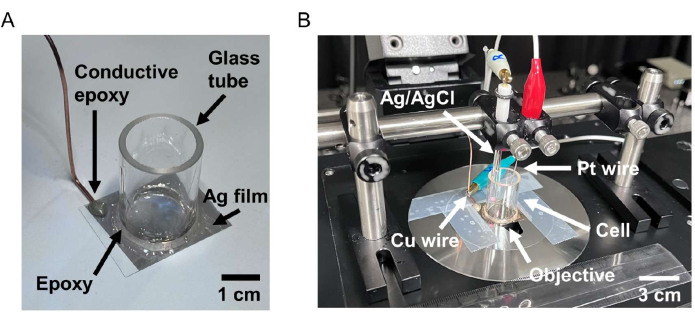
Figure 4: Preparation of the spectroelectrochemical cell. (A) A representative spectroelectrochemical cell prepared using a gap-mode SERS substrate as the working electrode. (B) A spectroelectrochemical cell immobilized on an inverted optical microscope stage for single-nanoparticle electrochemical spectroscopy and microscopy experiments. Please click here to view a larger version of this figure.
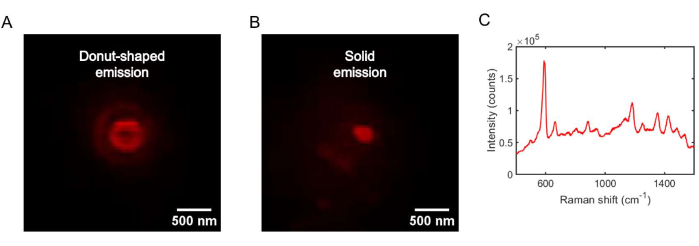
Figure 5: Identification of a single Ag nanoparticle on the Ag thin film substrate. (A) A donut-shaped NB SERS emission pattern, indicating that the signal originates from an individual Ag nanoparticle. (B) A solid NB SERS emission pattern, indicating that the signal originates from more than a single nanoparticle. (C) The SERS spectrum of the donut-shaped emission shown in (A), showing the characteristic peak at 592/cm from the ring deformation vibrational mode of NB52. Please click here to view a larger version of this figure.
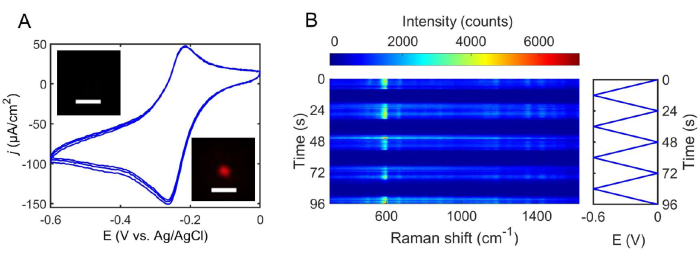
Figure 6: Electrochemistry and spectroelectrochemistry of NB. (A) Cyclic voltammograms of 0.5 mM NB in 0.1 M phosphate buffer (pH = 5) using an Ag disk working electrode. The insets show electrochemical SERS images of NB on an individual Ag nanoparticle on the gap-mode SERS substrate at the NB oxidation (bottom image) and reduction (top image) potentials. The scale bars represent 300 nm. (B) Electrochemical modulation of the NB SERS spectrum by cyclic voltammetry on a single Ag nanoparticle on the gap-mode SERS substrate. A Pt wire and an Ag/AgCl (3 M KCl) electrode were used as the counter and reference electrodes, respectively. Please click here to view a larger version of this figure.
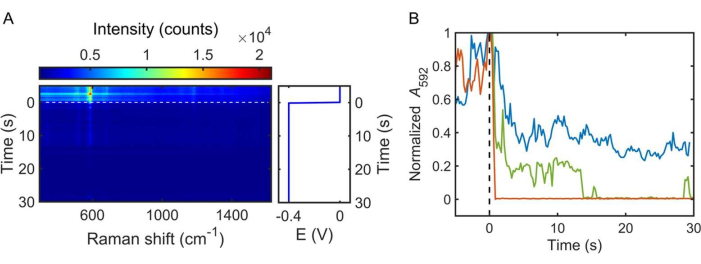
Figure 7: Potential step spectroelectrochemistry of NB. (A) Electrochemical modulation of the NB SERS spectrum by a potential step from 0 to −0.4 V (vs. Ag/AgCl) applied at t = 0 (dashed line). The intensity of the peak at 592/cm decreases with time due to the reduction of the NB molecules near the Ag nanoparticle. (B) Transient profile of the normalized area under the 592/cm peak as a function of the applied potential: −0.2 V (blue curve), −0.4 V (green curve), and −0.6 V (red curve). Please click here to view a larger version of this figure.
Discussion
Depositing Cu and Ag thin metal films on clean coverslips is vital to ensure that the final film has a roughness no greater than two to four atomic layers (or a root mean square roughness less than or equal to around 0.7 nm). Dust, scratches, and debris present on the coverslip prior to metal deposition are common issues that prevent the fabrication of the smooth film required to produce donut-shaped emission patterns. Hence, it is recommended to sonicate the coverslips in different solvents before the metal deposition and, if possible, to perform this process in a cleanroom. Further, careful attention should be paid to the deposition procedure. It may be necessary to clean all the surfaces inside the vacuum chamber (including the crucible holder drawer) and the crucibles, since these parts tend to accumulate dust and debris.
The high deposition rates used during the metal deposition process allow for the deposited film to be atomically smooth but may also be more difficult to control. The incorrect reading of the film thickness sensors may lead to inhomogeneous, excessively thick, or excessively thin films. If the metal films are too thin, islands of material may be deposited instead of a continuous surface. Films that are too thick will result in substrates that are opaque, which will prevent the excitation light from efficiently exciting the NB molecules and impede the collection of emission light; this, in turn, will decrease the overall sensitivity of the method and yield poor-quality SERS images and spectra with low signal-to-noise ratios. The deposition of Cu prior to Ag is crucial for the adhesion of the latter metal, but depositing excess Cu will reduce the optical transparency of the substrate, while an insufficient amount of Cu will lead to the delamination of the Ag from the glass coverslips. Further, if the sample platen's dimensions are greater than those of the shutter, evaporated metal may deposit on the coverslips while the shutter is closed, resulting in inhomogeneity of the substrate, as shown in Figure 2A.
The concentrations and incubation times for the NB solutions and Ag nanoparticle suspensions play a key role in producing a good-quality gap-mode SERS substrate. The use of NB solutions with concentrations higher than recommended in the protocol or the use of longer incubation times can lead to high background signals and, thus, pose challenges to locating individual Ag nanoparticles. On the other hand, a low NB solution concentration and a short incubation time will lead to low coverage of the NB molecules on the Ag thin film, which will make identifying single Ag nanoparticles a time-consuming process. Similarly, the use of an Ag nanoparticle suspension with a concentration higher than recommended in the protocol or the use of longer incubation times will lead to agglomeration of the Ag nanoparticles on the Ag thin film; this agglomeration will, thus, lead to SERS substrates that produce a high percentage of solid emission patterns and a decrease in the number of substrate sites that can be identified as single nanoparticles. In contrast, the use of a lower-concentration Ag nanoparticle suspension or a shorter incubation time will lead to a low coverage of Ag nanoparticles. In this case, a greater fraction of the SERS emission patterns will originate from single Ag nanoparticles, but the throughput of the experiment will be reduced.
For the successful implementation of the single-nanoparticle electrochemical SERS imaging and spectroscopy technique described in this paper, special attention needs to be paid to the spectroelectrochemical experimental setup. First, the identification of single Ag nanoparticles on the gap-mode SERS substrate using donut-shaped emission patterns is central for the successful use of the described method. Greater than 100x magnification of the optical image and a microscope objective with a high numerical aperture (e.g., 1.45) are typically required to observe the donut-shaped emission patterns. The high numerical aperture is particularly important for collecting high-angle emissions. Second, it is important to synchronize the collection of the SERS spectra with the electrochemical program. In this protocol, a transistor-transistor logic pulse is sent from the spectrometer detector to the potentiostat to trigger the simultaneous collection of the SERS spectra and electrochemical data. In addition, the readout time of the detector must be considered to accurately correlate the applied potentials with the SERS spectra in voltammetry.
The interpretation of vibrational modes is an important component of SERS spectroscopy. Ag is prone to forming oxides, which may affect the electrochemical process under study56. No oxide layers were detected by SERS in this protocol, but prolonged air exposure or oxidizing potentials cause the formation of oxides on the Ag mirror and/or nanoparticles. The oxide layers may alter the adsorption of redox-active molecules, thus inducing shifts in the vibrational modes. In the current protocol, we did not observe any shifts in vibrational modes between the NB molecules adsorbed onto the Si, the Ag film, or the Ag nanoparticles. We also note that the excitation of plasmon resonances, such as those of the gap-mode substrate, results in the generation of non-equilibrium hot electrons and hot holes that can participate in redox reactions57,58,59,60. To minimize interference from light-induced hot charge carriers, low light fluences are encouraged.
The technique described here can address the limitations of other single-nanoparticle techniques such as collision-based electrochemistry61,62,63, scanning electrochemical microscopy64,65,66, and scanning electrochemical cell microscopy67,68,69. It is possible to measure the electrochemical response of single nanoparticles using these electrochemical techniques; however, it is not possible to directly obtain the identity of and structural information about the reactants, intermediates, and products. The technique described in this protocol allows for tracking electrochemical reactions on single nanoparticles and obtaining chemical information through vibrational spectroscopy. However, this gap-mode SERS electrochemical method yields the best results when the SERS substrates are prepared using the most SERS-active metals under visible light excitation: Ag and Au. This may limit the choice of metals that can be employed in the technique. Further, while gap-mode SERS provides chemical information on electrochemical processes occurring on single nanoparticles, it only yields ensemble-averaged electrochemical information, as the current response is measured over the entire substrate. Nevertheless, the technique demonstrated in this paper is a powerful tool that can be used to gain fundamental mechanistic knowledge in diverse areas of electrochemistry, including in the fields of electrocatalytic reactions, which are important for energy storage70,71, chemical feedstock synthesis72,73, and sensors74,75.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by start-up funds from the University of Louisville and funding from Oak Ridge Associated Universities through a Ralph E. Powe Junior Faculty Enhancement Award. The authors thank Dr. Ki-Hyun Cho for creating the image in Figure 1. The metal deposition and SEM were performed at the Micro/Nano Technology Center at the University of Louisville.
Materials
| Acetone, microelectronic grade | J. T. Baker | 9005-05 | |
| Adjustable pipette, Eppendorf Reference 2 5000 mL | Eppendorf | 4924000100 | |
| Analytical Balance, AB54-S/FACT | Metter Toledo | N.A. | |
| Atomic Force Microscope, Easy scan 2 | Nanosurf | N.A. | |
| AXXIS Electron Beam Thin Film Deposition System | Kurt J. Lesker | N.A. | |
| Cary 60 UV-Vis Spectrophotometer | Agilent | N.A. | |
| Conductive epoxy, two part | Electron Microscopy Sciences | 12642-14 | |
| Copper pellets, 99.99% pure | Kurt J. Lesker | EVMCU40EXE | |
| Copper wire, bare, 18 AWG | VWR | 66248-040 | |
| Crucible, Graphite E-Beam | Kurt J. Lesker | EVCEB-23 | |
| Diamond Scriber | Ted Pella | 54484 | |
| EMCCD Camera, ProEM HS: 1024BX3 | Teledyne Princeton Instruments | N.A. | |
| Epoxy, Clear | Gorilla Glue | N.A. | |
| Glass Tube Cutter | Wheeler-Rex | 69012 | |
| Glass Tube, Borossilicate (OD 0.75", ID 0.62", L 12") | McMaster-Carr | 8729K45 | |
| Immersion oil, Type-F | Olympus | IMMOIL-F30CC | |
| Inverted Microscope, IX73 | Olympus | N.A. | |
| Laser, Excelsior One 642 nm Free space | Spectra-Physics | N.A. | |
| LightField | Teledyne Princeton Instruments | N.A. | |
| MATLAB 2022b | MathWorks | N.A. | |
| Micro cover glass (coverslips), 24×60 mm No. 1 | VWR | 48404-455 | |
| Microscope Smartphone Camera Adapter | qhma | QHMC017A-S01 | |
| Nile Blue A, pure | Acros Organics | 415690100 | |
| Nitrogen, Ultra Pure, Compressed | Specialty Gases | N.A. | |
| Objective, UPLanXApo 100× Oil Immersion | Olympus | 14-910 | |
| Polyimide Film, Kapton | 3M | 16089-4 | |
| Potassium Phosphate Monobasic | VWR | P285 | |
| Potentiostat, 660E | CH Instruments | N.A. | |
| Pt wire | Alfa Aesar | 10956-BS | |
| Scanning Electron Microscope, Apreo C SEM | Thermo Fischer Scientific | N.A. | |
| Si wafer | Ted Pella | 16006 | |
| Silver nanoparticles (nanospheres), NanoXact 0.02 mg/mL in 2 mM citrate | nanoComposix | AGCN60 | |
| Silver pellets, 99.99% pure | Kurt J. Lesker | EVMAG40EXE-A | |
| Slide Rack, Wash-N-Dry | Diversified Biotech | WSDR-2000 | |
| Smartphone, iPhone 13 mini | Apple | N.A. | |
| Sodium Phosphate Dibasic Heptahydrate | VWR | 0348 | |
| Spectrometer, IsoPlane SCT320 | Teledyne Princeton Instruments | N.A. | |
| Tissue Wipers, Light-duty | VWR | 82003-820 | |
| Tweezers, KS-04 | Kaisi Hardware | N.A. | |
| Utrasonic Generator, sweepSONIK | Blackstone-NEY Ultrasonics | 809379 | |
| Water Ultrapurifier, Sartorius Arium mini | Sartorius | N.A. |
References
- O’Mari, O., Vullev, V. I. Electrochemical analysis in charge-transfer science: The devil in the details. Current Opinion in Electrochemistry. 31, 100862 (2022).
- Forster, R. J. Microelectrodes: New dimensions in electrochemistry. Chemical Society Reviews. 23 (4), 289-297 (1994).
- Frackowiak, E., Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon. 39 (6), 937-950 (2001).
- Bard, A. J., Faulkner, L. R. . Electrochemical Methods: Fundamentals and Applications. , (2001).
- Gerischer, H. The impact of semiconductors on the concepts of electrochemistry. Electrochimica Acta. 35 (11), 1677-1699 (1990).
- Savéant, J. -. M. Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chemical Reviews. 108 (7), 2348-2378 (2008).
- Maduraiveeran, G., Sasidharan, M., Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosensors and Bioelectronics. 103, 113-129 (2018).
- Baker, L. A. Perspective and prospectus on single-entity electrochemistry. Journal of the American Chemical Society. 140 (46), 15549-15559 (2018).
- Wang, Y., Shan, X., Tao, N. Emerging tools for studying single entity electrochemistry. Faraday Discussions. 193, 9-39 (2016).
- Wilson, A. J., Willets, K. A. Visualizing site-specific redox potentials on the surface of plasmonic nanoparticle aggregates with superlocalization SERS microscopy. Nano Letters. 14 (2), 939-945 (2014).
- Devasia, D., Wilson, A. J., Heo, J., Mohan, V., Jain, P. K. A rich catalog of C-C bonded species formed in CO2 reduction on a plasmonic photocatalyst. Nature Communications. 12 (1), 2612 (2021).
- Sambur, J. B., et al. Sub-particle reaction and photocurrent mapping to optimize catalyst-modified photoanodes. Nature. 530 (7588), 77-80 (2016).
- Sambur, J. B., Chen, P. Approaches to single-nanoparticle catalysis. Annual Review of Physical Chemistry. 65 (1), 395-422 (2014).
- Sambur, J. B., Chen, P. Distinguishing direct and indirect photoelectrocatalytic oxidation mechanisms using quantitative single-molecule reaction imaging and photocurrent measurements. The Journal of Physical Chemistry C. 120 (37), 20668-20676 (2016).
- Wang, L., Tahir, M., Chen, H., Sambur, J. B. Probing charge carrier transport and recombination pathways in monolayer MoS2/WS2 heterojunction photoelectrodes. Nano Letters. 19 (12), 9084-9094 (2019).
- Rubin, H. The significance of biological heterogeneity. Cancer and Metastasis Reviews. 9 (1), 1-20 (1990).
- Altschuler, S. J., Wu, L. F. Cellular heterogeneity: Do differences make a difference. Cell. 141 (4), 559-563 (2010).
- Guerrette, J. P., Percival, S. J., Zhang, B. Fluorescence coupling for direct imaging of electrocatalytic heterogeneity. Journal of the American Chemical Society. 135 (2), 855-861 (2013).
- Chen, Y., et al. In situ imaging facet-induced spatial heterogeneity of electrocatalytic reaction activity at the subparticle level via electrochemiluminescence microscopy. Analytical Chemistry. 91 (10), 6829-6835 (2019).
- Zaleski, S., et al. Investigating nanoscale electrochemistry with surface- and tip-enhanced Raman spectroscopy. Accounts of Chemical Research. 49 (9), 2023-2030 (2016).
- Xu, R., et al. Heterogeneous damage in Li-ion batteries: Experimental analysis and theoretical modeling. Journal of the Mechanics and Physics of Solids. 129, 160-183 (2019).
- Liu, H., et al. Quantifying reaction and rate heterogeneity in battery electrodes in 3D through operando X-ray diffraction computed tomography. ACS Applied Materials & Interfaces. 11 (20), 18386-18394 (2019).
- Heinze, J. Ultramicroelectrodes in electrochemistry. Angewandte Chemie International Edition in English. 32 (9), 1268-1288 (1993).
- Arrigan, D. W. M. Nanoelectrodes, nanoelectrode arrays and their applications. Analyst. 129 (12), 1157-1165 (2004).
- Grall, S., et al. Attoampere nanoelectrochemistry. Small. 17 (29), 2101253 (2021).
- Sa, N., Lan, W. -. J., Shi, W., Baker, L. A. Rectification of ion current in nanopipettes by external substrates. ACS Nano. 7 (12), 11272-11282 (2013).
- Zhu, C., Huang, K., Siepser, N. P., Baker, L. A. Scanning ion conductance microscopy. Chemical Reviews. 121 (19), 11726-11768 (2021).
- Fu, K., Kwon, S. -. R., Han, D., Bohn, P. W. Single entity electrochemistry in nanopore electrode arrays: Ion transport meets electron transfer in confined geometries. Accounts of Chemical Research. 53 (4), 719-728 (2020).
- Iwasita, T., Nart, F. C., Rodes, A., Pastor, E., Weber, M. Vibrational spectroscopy at the electrochemical interface. Surface Structure and Electrochemical Reactivity. 40 (1), 53-59 (1995).
- Joshi, P. B., Wilson, A. J. Understanding electrocatalysis at nanoscale electrodes and single atoms with operando vibrational spectroscopy. Current Opinion in Green and Sustainable Chemistry. 38, 100682 (2022).
- Kaim, W., Fiedler, J. Spectroelectrochemistry: The best of two worlds. Chemical Society Reviews. 38 (12), 3373-3382 (2009).
- Zhai, Y., Zhu, Z., Zhou, S., Zhu, C., Dong, S. Recent advances in spectroelectrochemistry. Nanoscale. 10 (7), 3089-3111 (2018).
- Zheng, X., Zong, C., Xu, M., Wang, X., Ren, B. Raman imaging from microscopy to nanoscopy, and to macroscopy. Small. 11 (28), 3395-3406 (2015).
- Opilik, L., Schmid, T., Zenobi, R. Modern Raman imaging: Vibrational spectroscopy on the micrometer and nanometer scales. Annual Review of Analytical Chemistry. 6 (1), 379-398 (2013).
- Wilson, A. J., Devasia, D., Jain, P. K. Nanoscale optical imaging in chemistry. Chemical Society Reviews. 49 (16), 6087-6112 (2020).
- Willets, K. A. Probing nanoscale interfaces with electrochemical surface-enhanced Raman scattering. Current Opinion in Electrochemistry. 13, 18-24 (2019).
- Tian, Z. -. Q., Ren, B. Adsorption and reaction at electrochemical interfaces as probed by surface-enhanced Raman spectroscopy. Annual Review of Physical Chemistry. 55 (1), 197-229 (2004).
- Wu, D. -. Y., Li, J. -. F., Ren, B., Tian, Z. -. Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chemical Society Reviews. 37 (5), 1025-1041 (2008).
- Bosnick Jiang, K., Maillard, M., Brus, L. Single molecule Raman spectroscopy at the junctions of large Ag nanocrystals. The Journal of Physical Chemistry B. 107 (37), 9964-9972 (2003).
- Camden, J. P., et al. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. Journal of the American Chemical Society. 130 (38), 12616-12617 (2008).
- Daniels, J. K., Chumanov, G. Nanoparticle−mirror sandwich substrates for surface-enhanced Raman scattering. The Journal of Physical Chemistry B. 109 (38), 17936-17942 (2005).
- Ciracì, C., et al. Probing the ultimate limits of plasmonic enhancement. Science. 337 (6098), 1072-1074 (2012).
- Ausman, L. K., Schatz, G. C. On the importance of incorporating dipole reradiation in the modeling of surface enhanced Raman scattering from spheres. The Journal of Chemical Physics. 131 (8), 084708 (2009).
- Stranahan, S. M., Willets, K. A. Super-resolution optical imaging of single-molecule SERS hot spots. Nano Letters. 10 (9), 3777-3784 (2010).
- Titus, E. J., Weber, M. L., Stranahan, S. M., Willets, K. A. Super-resolution SERS imaging beyond the single-molecule limit: An isotope-edited approach. Nano Letters. 12 (10), 5103-5110 (2012).
- Bartko, A. P., Dickson, R. M. Imaging three-dimensional single molecule orientations. The Journal of Physical Chemistry B. 103 (51), 11237-11241 (1999).
- Chen, S. -. Y., et al. Gold nanoparticles on polarizable surfaces as Raman scattering antennas. ACS Nano. 4 (11), 6535-6546 (2010).
- Du, L., Tang, D., Yuan, G., Wei, S., Yuan, X. Emission pattern of surface-enhanced Raman scattering from single nanoparticle-film junction. Applied Physics Letters. 102 (8), 081117 (2013).
- Joshi, P. B., Anthony, T. P., Wilson, A. J., Willets, K. A. Imaging out-of-plane polarized emission patterns on gap mode SERS substrates: From high molecular coverage to the single molecule regime. Faraday Discussions. 205, 245-259 (2017).
- Stranahan, S. M., Titus, E. J., Willets, K. A. SERS orientational imaging of silver nanoparticle dimers. The Journal of Physical Chemistry Letters. 2 (21), 2711-2715 (2011).
- Cortés, E., et al. Monitoring the electrochemistry of single molecules by surface-enhanced Raman spectroscopy. Journal of the American Chemical Society. 132 (51), 18034-18037 (2010).
- Wilson, A. J., Molina, N. Y., Willets, K. A. Modification of the electrochemical properties of Nile Blue through covalent attachment to gold as revealed by electrochemistry and SERS. The Journal of Physical Chemistry C. 120 (37), 21091-21098 (2016).
- E-beam evaporator SOP. Micro/Nano Technology Center, University of Louisville Available from: https://louisville.edu/micronano/files/documents/standard-operating-procedures/Ebaeam_SOP.pdf (2020)
- FEI Apreo C SEM SOP. Micro/Nano Technology Center, University of Louisville Available from: https://louisville.edu/micronano/files/documents/standard-operating-procedures/ApreoSEMSOPn.pdf (2023)
- Benz, F., et al. SERS of individual nanoparticles on a mirror: Size does matter, but so does shape. The Journal of Physical Chemistry Letters. 7 (12), 2264-2269 (2016).
- Sundaresan, V., Monaghan, J. W., Willets, K. A. Visualizing the effect of partial oxide formation on single silver nanoparticle electrodissolution. The Journal of Physical Chemistry C. 122 (5), 3138-3145 (2018).
- Wilson, A. J., Mohan, V., Jain, P. K. Mechanistic understanding of plasmon-enhanced electrochemistry. The Journal of Physical Chemistry C. 123 (48), 29360-29369 (2019).
- Wilson, A. J., Jain, P. K. Light-induced voltages in catalysis by plasmonic nanostructures. Accounts of Chemical Research. 53 (9), 1773-1781 (2020).
- Wang, J., Heo, J., Chen, C., Wilson, A. J., Jain, P. K. Ammonia oxidation enhanced by photopotential generated by plasmonic excitation of a bimetallic electrocatalyst. Angewandte Chemie International Edition. 59 (42), 18430-18434 (2020).
- Joshi, P. B., Wilson, A. J. Plasmonically enhanced electrochemistry boosted by nonaqueous solvent. The Journal of Chemical Physics. 156 (24), 241101 (2022).
- Xiao, X., Fan, F. -. R. F., Zhou, J., Bard, A. J. Current transients in single nanoparticle collision events. Journal of the American Chemical Society. 130 (49), 16669-16677 (2008).
- Kwon, S. J., et al. Stochastic electrochemistry with electrocatalytic nanoparticles at inert ultramicroelectrodes-theory and experiments. Physical Chemistry Chemical Physics. 13 (12), 5394-5402 (2011).
- Anderson, T. J., Zhang, B. Single-nanoparticle electrochemistry through immobilization and collision. Accounts of Chemical Research. 49 (11), 2625-2631 (2016).
- Sun, T., Yu, Y., Zacher, B. J., Mirkin, M. V. Scanning electrochemical microscopy of individual catalytic nanoparticles. Angewandte Chemie International Edition. 53 (51), 14120-14123 (2014).
- Yu, Y., Sun, T., Mirkin, M. V. Scanning electrochemical microscopy of single spherical nanoparticles: Theory and particle size evaluation. Analytical Chemistry. 87 (14), 7446-7453 (2015).
- Yu, Y., et al. Electrochemistry and electrocatalysis at single gold nanoparticles attached to carbon nanoelectrodes. ChemElectroChem. 2 (1), 58-63 (2015).
- Bentley, C. L., Kang, M., Unwin, P. R. Nanoscale structure dynamics within electrocatalytic materials. Journal of the American Chemical Society. 139 (46), 16813-16821 (2017).
- Wahab, O. J., Kang, M., Unwin, P. R. Scanning electrochemical cell microscopy: A natural technique for single entity electrochemistry. Current Opinion in Electrochemistry. 22, 120-128 (2020).
- Bentley, C. L., et al. Local surface structure and composition control the hydrogen evolution reaction on iron nickel sulfides. Angewandte Chemie International Edition. 57 (15), 4093-4097 (2018).
- Wright, D., et al. Mechanistic study of an immobilized molecular electrocatalyst by in situ gap-plasmon-assisted spectro-electrochemistry. Nature Catalysis. 4 (2), 157-163 (2021).
- Peng, J., et al. In-situ spectro-electrochemistry of conductive polymers using plasmonics to reveal doping mechanisms. ACS Nano. 16 (12), 21120-21128 (2022).
- Yan, M., Kawamata, Y., Baran, P. S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chemical Reviews. 117 (21), 13230-13319 (2017).
- Kingston, C., et al. A survival guide for the "electro-curious.". Accounts of Chemical Research. 53 (1), 72-83 (2020).
- Patrice, F. T., Qiu, K., Ying, Y. -. L., Long, Y. -. T. Single nanoparticle electrochemistry. Annual Review of Analytical Chemistry. 12 (1), 347-370 (2019).
- Sekretareva, A. Single-entity electrochemistry of collision in sensing applications. Sensors and Actuators Reports. 3, 100037 (2021).

