Quantification, Viability Assessment, and Visualization Strategies for Acinetobacter Biofilms
Summary
This protocol describes the preparation of the inoculum, the biofilm quantification on microtiter plates using crystal violet dye, the viable count in biofilms, and the visualization of biofilms of Acinetobacter.
Abstract
Acinetobacter causes nosocomial infections and its biofilm formation can contribute to the survival on dry surfaces such as hospital environments. Thus, biofilm quantification and visualization are important methods to assess the potential of Acinetobacter strains to cause nosocomial infections. The biofilms forming on the surface of the microplate can be quantified in terms of volume and cell numbers. Biofilm volumes can be quantified by staining using crystal violet, washing, destaining using ethanol, then measuring the solubilized dye using a microplate reader. To quantify the number of cells embedded in the biofilms, the biofilms are scrapped off using cell scrapers, harvested in the saline, vigorously agitated in the presence of glass beads, and spread on Acinetobacter agar. Then, the plates are incubated at 30 °C for 24-42 h. After incubation, the red colonies are enumerated to estimate the number of cells in biofilms. This viable count method can also be useful for counting Acinetobacter cells in mixed-species biofilms. Acinetobacter biofilms can be visualized using fluorescent dyes. A commercially available microplate designed for microscopic analysis is employed to form biofilms. Then, the bottom-surface attached biofilms are stained with SYTO9 and propidium iodide dyes, washed, then visualized with confocal laser scanning microscopy.
Introduction
Acinetobacter is known to cause nosocomial infections, and its human infection, especially in healthcare facilities, is increasingly reported1. It is widespread in hospitals, healthcare facilities, and food-associated environments2,3,4. It can survive for a long period in environments including hospital surfaces such as bed rails, bedside tables, the surface of ventilators, and sinks4. Such persistence on environmental surfaces may be one of the significant factors contributing to the nosocomial infections of Acinetobacter4.
Biofilm is a form of microbial life and is a microbial matrix composed of live microbial cells and extracellular polymeric substances (EPS) from the cells5. The microbial cells are embedded in the matrix and are often highly resistant to environmental stresses such as heat, salts, dryness, antibiotics, disinfectants, and shear forces6,7.
Acinetobacter can form biofilms on surfaces, suggesting that it may contribute to extended survival on environmental surfaces, including hospital surfaces, and enhanced resistance to antibiotic treatment8,9. In addition, the biofilm formation of Acinetobacter could be highly associated with human clinical outcomes8. Therefore, the biofilm formability of Acinetobacter strains could be one of the indicators in predicting environmental survival and human infections8,10.
The surface-attached biofilms can be quantified and visualized to assess biofilm formability. To quantify surface-attached biofilms, the biofilms are normally stained by biofilm-staining dyes such as crystal violet, and the dyes are eluted in solution and measured for optical density11. Visualization of biofilms is another good approach to assess biofilm formability11. Confocal Laser Scanning Microscopy (CLSM) visualization method employing specificity using fluorescent dyes could be more useful to characterize biofilm morphology compared to other techniques such as SEM12,13.
The viable cells in biofilms can be counted to estimate the number of viable cells in biofilms11. The viable cells embedded in biofilms are detached, diluted, spread on agar plates, incubated, and enumerated. Because the higher number of cells is likely to meet the infectious dose, it can provide more detailed information on biofilms, such as infection potentials associated with the number of cells13,14.
This article presents step-by-step protocols to (1) quantify surface-attached biofilms, (2) count viable cells in the biofilms, and (3) visualize the biofilms using CLSM of Acinetobacter. The presented protocols describe the methods to assess the biofilm formability of Acinetobacter isolates and characterize their biofilms.
Protocol
1. Preparation of bacterial inoculum
- Remove the glycerol stock vial stored at -80 °C.
- Remove the bacterial strain (2-10 µL) from the vial using a sterile pipette tip.
NOTE: Acinetobacter strains, A. bouvetii (13-1-1), A. junii (13-1-2), A. pittii (13-2-5), A. baumannii (13-2-9), A. radioresistens (20-1), and A. ursingii (24-1) are used in this protocol. - Inoculate a commercially available blood agar plate (tryptic soy agar supplemented with 5% sheep blood, see Table of Materials) with the bacteria.
NOTE: Other media, such as nutrient agar or MacConkey agar, can also be used. - Streak the agar surface using a sterile loop with intermittent flaming to thin it out.
- Place the agar plate upside down in an incubator and incubate at 30 °C overnight for 20-24 h.
- Pick a single colony of the bacterial culture with a sterile needle and inoculate 5 mL sterile brain heart infusion broth (BHI, see Table of Materials) with the culture.
- Incubate the inoculated broth in a shaking incubator at 30 °C overnight for 20-24 h at around 150 rpm.
- Transfer 1 mL of the overnight culture to a sterile microcentrifuge tube and centrifuge the overnight culture at 6,000 × g for 10 min at room temperature (RT).
- Remove the supernatant with a pipette.
- Using a vortex, resuspend the pellet in 1 mL sterile phosphate-buffered saline (PBS).
- Centrifuge at 6,000 × g for 10 min at RT and remove the supernatant.
- Resuspend the pellet in sterile ten-fold diluted BHI using vortex to an optical density of around 0.1 at 600 nm.
NOTE: The optical density of around 0.1 is equivalent to approximately 107 CFU/mL.
2. Biofilm quantification using crystal violet
- Add 200 µL inoculum to each well of a sterile polystyrene 96-well plate (see Table of Materials) and add 200 µL deionized water to each of the outermost wells to prevent the inner wells from drying out15.
- Add 200 µL ten-fold diluted BHI to each well of the same plate as a negative control.
- Incubate the plate at 25 °C for 24 h.
- Remove the supernatant with a pipette.
NOTE: Multichannel pipette is often more convenient for multiple samples. - Carefully add 300 µL PBS to each well and remove the supernatant with a pipette.
NOTE: Multichannel pipette is often more convenient for multiple samples. - Repeat step 2.5 two more times.
- Add 200 µL crystal violet solution (1%) (see Table of Materials) to each well and incubate at RT for 30 min.
- Repeat steps 2.4-2.6.
- Add 200 µL of absolute ethanol to each well and incubate for 15 min at RT. Mix thoroughly by pipetting up and down several times.
- Transfer 100 µL elution from each well to a new 96-well plate.
- Measure the absorbance at 595 nm using a microplate reader (see Table of Materials).
- When the absorbance exceeds 2.0, make a proper dilution of the sample to the absorbance below 2.0 in absolute ethanol and measure it as in step 2.11. Then, calculate the absorbance of the sample (final value) by multiplying the observed value by the dilution factor.
- Calculate the true absorbance of the samples by subtracting the average value of the negative control from the value of each well of the test samples on the same well plate.
3. Biofilm viability count
- Add 1 mL inoculum (step 1) to each well of a sterile polystyrene 12-well plate15. Incubate the plate at 25 °C for 24 h. Remove the supernatant with a pipette.
- Carefully add 1.5 mL sterile PBS to each well and remove the supernatant with a pipette. Add 1 mL sterile PBS to each well.
- Scrape off the bottom and wall surfaces of the well using fitted cell scrapers.
- Transfer the harvested cell suspension to a sterile plastic tube (10-1) containing 9 mL saline (0.85% NaCl) and 10-20 glass beads (see Table of Materials).
NOTE: To collect any residual biofilm debris left on the bottom surface of the well, it can be resuspended and transferred by pipette using saline from the 10-1 tube. - Vortex the 10-1 tube for 60 s at a maximum speed.
- Make a 10-fold serial dilution by transferring 1 mL to the next sterile tube (10-2) containing 9 mL saline (0.85 % NaCl) up to 10-7.
- Spread 100 µL from each dilution on Acinetobacter agar (see Table of Materials) and incubate the agar plates at 30 °C for 24-42 h.
- Count the number of the typical red colonies on each plate manually in the range between 10 and 250.
- Calculate the number of viable cells in the biofilm of each well by using the equation below:
Viable cells = Number of colonies × Dilution factors × 10
4. Biofilm visualization using confocal laser scanning microscopy (CLSM)
- Add 200 µL inoculum to each well of a sterile 96-well plate intended for microscopic analysis.
- Incubate the plate at 25 °C for 24 h.
- Remove the supernatant with a pipette.
- Carefully add 300 µL filter-sterilized deionized water to each well and remove the supernatant with a pipette.
- Repeat step 4.4.
- Prepare the mixture of SYTO 9 and propidium iodide (see Table of Materials) diluted in filter-sterilized deionized water to a final concentration of 10 µM and 60 µM, respectively.
- Add the mixture to each well at 200 µL and incubate the plate for 20-30 min at RT in the dark.
- Repeat steps 4.3-4.4
- Visualize the bottom-surface attached biofilms using CLSM with the excitation wavelength of 488 nm and 561 nm and the emission wavelength range of 499-535 nm and 568-712 nm, respectively.
Representative Results
Following the protocol, the biofilms of Acinetobacter isolates, originally isolated from kitchen surfaces, were formed on a polystyrene 96-well plate, stained with crystal violet, and the dyes were solubilized in ethanol and measured for biofilm mass (Figure 1). The number of biofilms greatly varied depending on the strains ranging from OD 0.04 to 1.69 (Figure 1). Based on the criteria established by Stepanović et al.16, all of the isolates except for A. bouvetii formed biofilms. A. radioresistens formed a weak biofilm. A. junii and A. baumannii formed moderate biofilms, while A. pittii and A. ursingii formed strong biofilms.
To count viable cells in biofilms, the biofilms were scraped off using cell scrapers, vortexed at high speed, diluted, and spread on the Acinetobacter selective plates. After incubation, the number of colonies was counted to estimate the number of biofilm cells (Figure 2). All the isolates except for A. bouvetii had cells equivalent to 7-8 Log CFU in their biofilms. Consistent with the biofilm non-forming property shown by crystal violet assay, A. bouvetii had a much lower level of cell number at 4.4 Log CFU.
The bottom-surface attached biofilms were visualized using CLSM (Figure 3). A substantial amount of biofilm was found in A. junii, A. baumannii, and A. ursingii with distinct biofilm morphologies. While A. pittii was a strong biofilm former in crystal violet assay, it did not form much biofilm on the bottom surface.
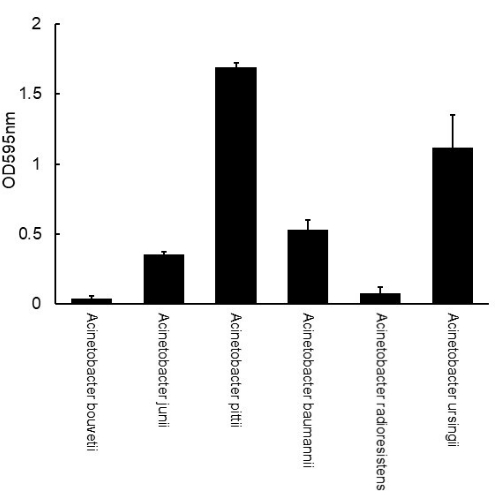
Figure 1: Measurement of biofilm mass of Acinetobacter formed on a polystyrene 96-well plate using crystal violet assay. The error bars represent the standard deviation from triplicate. Please click here to view a larger version of this figure.
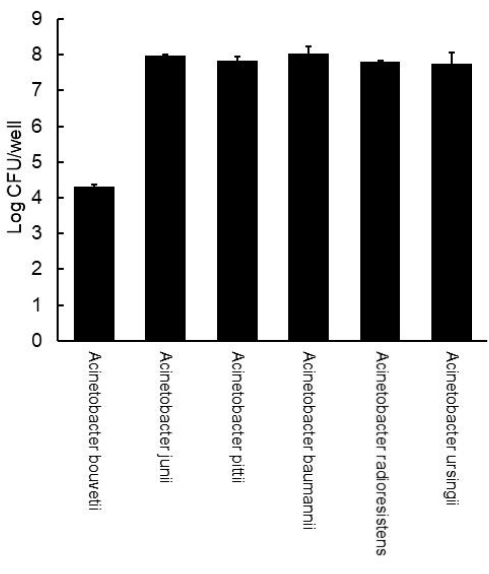
Figure 2: Viable counts of Acinetobacter biofilms formed on a polystyrene 12-well plate. The error bars represent the standard deviation from the duplicate. Please click here to view a larger version of this figure.
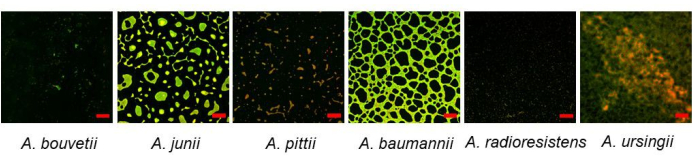
Figure 3: Bottom-surface attached Acinetobacter biofilms formed on a 96-well plate visualized by CLSM using SYTO 9 and propidium iodide dyes. Scale bars: 50 µm. Please click here to view a larger version of this figure.
Discussion
Using the protocol described, the biofilm formation of Acinetobacter isolates with varying degrees was measured, visualized, and the viable cells in the biofilms were estimated (Figure 1, Figure 2, and Figure 3).
In this protocol, two different temperatures were used, 30 °C for the growth and 25 °C for the biofilm formation of Acinetobacter. 30 °C was used because many studies used more than 30 °C for the optimal growth of Acinetobacter, which would be appropriate to obtain sufficient inoculum12,13,14,17. However, this temperature is generally higher than the temperatures of the environment where biofilm formation would occur, such as hospital surfaces or food processing environments. Therefore, 25 °C was used for biofilm formation, which would simulate these environmental conditions more.
This protocol used 10-fold diluted BHI instead of the original BHI to form biofilms (step 1.12). Many surfaces, such as hospital surfaces or food processing environments, are likely to be low-nutrient18,19. In addition, microorganisms on the contaminated surfaces remain on the surfaces after cleaning and persist in the low-nutrient conditions20. Therefore, low-nutrient conditions such as 10-fold diluted BHI are more desirable than high-nutrient conditions such as original BHI.
Crystal violet assay is a generally accepted and well-established method to measure biofilm mass, and this study showed that the biofilm formability of Acinetobacter is discernible at varying levels by using this method.
Viable count in biofilm is also important because the viable cells in biofilms are responsible for human infections that are more likely to occur by a higher number of cells21. In addition, the viable count may be a good tool to distinguish poor biofilm producers, as demonstrated in A. bouvetii and A. radioresistens (Figure 1 and Figure 2). The viable count of A. radioresistens was much higher than the one of A. bouvetii, while little difference was found in biofilm mass, suggesting a higher potential of infection by A. radioresistens (Figure 1 and Figure 2). Also, this method can be used to estimate the proportion of Acinetobacter in multi-species biofilms, which is more common in the environment.
Acinetobacter forms biofilm not only on the bottom surface but also on the sidewall, especially at the air-liquid interface in tubes17,22. CLSM analysis using a microtiter plate is limited to the bottom-surface attached biofilms, which makes it sometimes hard to compare to the results by crystal violet assay, which measures all the submerged surfaces, including the wall surfaces. A substantial amount of biofilm at the air-liquid interface was also found in these isolates, especially the strong biofilm producer, A. pittii. It formed relatively poor biofilm on the bottom surface compared to other isolates (Figure 3). Therefore, a microscopic analysis technique must be further developed to visualize the biofilms formed at the sidewall.
In this study, three different assays, crystal violet, viable count, and CLSM, were used to characterize the biofilms of Acinetobacter. Crystal violet assay is the well-established method to quantify biofilms, and the criteria to determine biofilm formability exist16. However, it measures not only live cells and EPS but also dead cells, which are less meaningful in terms of human infection and virulence. The live cells in biofilms can be measured by the viable count method. However, biofilm formability cannot be determined by the viable count method because this method does not measure EPS, a critical component in biofilm structure. Therefore, this method does not have the criteria or cut-off value to determine biofilm formability. In addition, the viable count method measures only the culturability of the cells and does not measure the cells under 'viable but not culturable state'11. The presence of biofilms can be confirmed by the CLSM method, which effectively visualizes biofilms. However, it measures only bottom surface-attached biofilms, although biofilms are often formed on the sidewalls as well. Therefore, using crystal violet assay to determine the biofilm formability is recommended. Then, the live and culturable cells in biofilms can be quantified by the viable count method, and the bottom surface-attached biofilms can be confirmed or characterized by the CLSM method.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Main Research Program (E0210702-03) of the Korea Food Research Institute (KFRI), funded by the Ministry of Science and ICT.
Materials
| 96-well cell culture plate | SPL | 30096 | Polystyrene 96-well plate |
| BHI (Brain Heart Infusion) broth | Merck KGaA | 1.10493.0500 | |
| Blood Agar Base Plate | KisanBio | MB-B1005-P50 | Growth media for Acinetobacter |
| CHROMagar Acinetobacter | CHROMagar | AC092 | Selective plate for Acinetobacter |
| Crystal violet solution | Sigma-Aldrich | V5265 | |
| Filmtracer LIVE/DEAD biofilm viability kit | Invitrogen | L10316 | SYTO9 and propidium iodide |
| Microplate reader | Tecan | Infinite M200 PRO NanoQuant | Biofilm measurement |
| RBC Glass Plating Beads | RBC | RG001 | Glass beads |
| μ-Plate 96 Well Black | ibidi | 89621 | Microplate intended for CLSM |
References
- Wong, D., et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clinical Microbiology Reviews. 30 (1), 409-447 (2017).
- Carvalheira, A., Silva, J., Teixeira, P. Acinetobacter spp. in food and drinking water – A review. Food Microbiology. 95, 103675 (2021).
- Towner, K. J. Acinetobacter: an old friend, but a new enemy. Journal of Hospital Infection. 73 (4), 355-363 (2009).
- Weber, D. J., Rutala, W. A., Miller, M. B., Huslage, K., Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. American Journal of Infection Control. 38 (5), S25-S33 (2010).
- Flemming, H. C., et al. The biofilm matrix: multitasking in a shared space. Nature Reviews Microbiology. 21 (2), 70-86 (2023).
- Flemming, H. C., et al. Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology. 14 (9), 563-575 (2016).
- Yin, W., Wang, Y., Liu, L., He, J. Biofilms: the microbial "protective clothing" in extreme environments. International Journal of Molecular Sciences. 20 (14), 3423 (2019).
- Gedefie, A., et al. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infection and drug resistance. 14, 3711-3719 (2021).
- Whiteway, C., Breine, A., Philippe, C., Van der Henst, C. Acinetobacter baumannii. Trends in Microbiology. 30 (2), 199-200 (2022).
- Longo, F., Vuotto, C., Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiologica. 37 (2), 119-127 (2014).
- Azeredo, J., et al. Critical review on biofilm methods. Critical Reviews in Microbiology. 43 (3), 313-351 (2017).
- Jia, J., Xue, X., Guan, Y., Fan, X., Wang, Z. Biofilm characteristics and transcriptomic profiling of Acinetobacter johnsonii defines signatures for planktonic and biofilm cells. Environmental Research. 213, 113714 (2022).
- Yang, C., Su, P., Moi, S., Chuang, L. Biofilm formation in Acinetobacter baumannii: genotype-phenotype correlation. Molecules. 24 (10), 1849 (2019).
- Alamri, A. M., Alsultan, A. A., Ansari, M. A., Alnimr, A. M. Biofilm-formation in clonally unrelated multidrug-resistant Acinetobacter baumannii isolates. Pathogens. 9 (8), 630 (2020).
- Lim, E. S., Nam, S. J., Koo, O. K., Kim, J. S. Protective role of Acinetobacter and Bacillus for Escherichia coli O157:H7 in biofilms against sodium hypochlorite and extracellular matrix-degrading enzymes. Food Microbiology. 109, 104125 (2023).
- Stepanović, S., Ćirković, I., Ranin, L., Svabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in Applied Microbiology. 38 (5), 428-432 (2004).
- Boone, R. L., et al. Analysis of virulence phenotypes and antibiotic resistance in clinical strains of Acinetobacter baumannii isolated in Nashville, Tennessee. BMC Microbiology. 21 (1), 21 (2021).
- Otter, J. A., et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. Journal of Hospital Infection. 89 (1), 16-27 (2015).
- Ravishankar, S., Juneja, V. K., Yousef, A. E., Juneja, V. K. Adaptation or resistance responses of microorganisms to stresses in the food processing environment. Microbial Stress Adaptation and Food Safety. , (2003).
- Dewanti, R., Wong, A. C. L. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. International Journal of Food Microbiology. 26 (2), 147-164 (1995).
- McConnell, M. J., Actis, L., Pachón, J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiology Reviews. 37 (2), 130-155 (2013).
- McQueary, C. N., Actis, L. A. Acinetobacter baumannii biofilms: variations among strains and correlations with other cell properties. The Journal of Microbiology. 49 (2), 243-250 (2011).

