Minimally Invasive Murine Laryngoscopy for Close-Up Imaging of Laryngeal Motion During Breathing and Swallowing
Summary
This protocol describes a serial transoral laryngoscopy approach for mice and rats that permits close-up, unobstructed video imaging of the larynx during breathing and swallowing using an optimized anesthetic regimen and finely tuned endoscopic manipulation techniques.
Abstract
The larynx is an essential organ in mammals with three primary functions – breathing, swallowing, and vocalizing. A wide range of disorders are known to impair laryngeal function, which results in difficulty breathing (dyspnea), swallowing impairment (dysphagia), and/or voice impairment (dysphonia). Dysphagia, in particular, can lead to aspiration pneumonia and associated morbidity, recurrent hospitalization, and early mortality. Despite these serious consequences, existing treatments for laryngeal dysfunction are largely aimed at surgical and behavioral interventions that unfortunately do not typically restore normal laryngeal function, thus highlighting the urgent need for innovative solutions.
To bridge this gap, we have been developing an experimental endoscopic approach to investigate laryngeal dysfunction in murine (i.e., mouse and rat) models. However, endoscopy in rodents is quite challenging due to their small size relative to current endoscope technology, anatomical differences in the upper airway, and the necessity for anesthesia to optimally access the larynx. Here, we describe a novel transoral laryngoscopy approach that permits close-up, unobstructed video imaging of laryngeal motion in mice and rats. Critical steps in the protocol include precise anesthesia management (to prevent overdosing that abolishes swallowing and/or risks respiratory distress-related mortality) and micromanipulator control of the endoscope (for stable video recording of laryngeal motion by a single researcher for subsequent quantification).
Importantly, the protocol can be performed over time in the same animals to study the impact of various pathological conditions specifically on laryngeal function. A novel advantage of this protocol is the ability to visualize airway protection during swallowing, which is not possible in humans due to epiglottic inversion over the laryngeal inlet that obstructs the glottis from view. Rodents therefore provide a unique opportunity to specifically investigate the mechanisms of normal versus pathological laryngeal airway protection for the ultimate purpose of discovering treatments to effectively restore normal laryngeal function.
Introduction
The larynx is a cartilaginous organ located at the intersection of the respiratory and digestive tracts in the throat, where it functions as a valving mechanism to precisely control the flow and direction of air (i.e., during breathing and vocalizing) versus food and liquid (i.e., during swallowing). A wide range of disorders are known to affect the larynx, including congenital (e.g., laryngomalacia, subglottic stenosis), neoplastic (e.g., laryngeal papillomatosis, squamous cell carcinoma), neurological (e.g., idiopathic laryngeal paralysis, stroke, Parkinson's disease, amyotrophic lateral sclerosis), and iatrogenic (e.g., inadvertent injury during head or neck surgery). Regardless of the etiology, laryngeal dysfunction typically results in a symptom triad of dyspnea (difficulty breathing), dysphonia (voice impairment), and dysphagia (swallowing impairment) that negatively impact a person's economic and social welfare1,2,3,4.
Moreover, dysphagia, particularly in medically fragile individuals, can lead to aspiration pneumonia (due to food or liquid escaping through an incompletely closed larynx into the lungs) and associated morbidity, recurrent hospitalization, and early mortality5,6. Despite these serious consequences, existing treatments for laryngeal dysfunction are largely aimed at surgical and behavioral interventions that do not typically restore normal laryngeal function1,2,7,8,9,10, thus highlighting the urgent need for innovative solutions. Toward this goal, we have been developing an experimental endoscopic approach to investigate laryngeal dysfunction in murine (i.e., mouse and rat) models.
In human medicine, the gold standard for the evaluation of laryngeal dysfunction is endoscopic visualization, referred to as laryngoscopy11,12. Typically, a flexible endoscope is passed through the nose to examine the larynx, particularly the vocal folds and adjacent supraglottic and subglottic laryngeal structures. A rigid endoscope may also be used to visualize the larynx via the oral cavity. Either approach permits gross examination of laryngeal anatomy and can be used to assess laryngeal mobility and function during respiration, phonation, and a variety of airway protective reflexes such as coughing and the laryngeal adductor reflex13,14,15,16. During swallowing, however, the larynx is completely obscured by the epiglottis as it inverts to cover the laryngeal entrance, protecting it from the path of the food/liquid bolus being swallowed. As a result, direct visualization of laryngeal motion during swallowing is not possible in humans and must therefore be indirectly inferred using other diagnostic approaches (e.g., fluoroscopy, electromyography, electroglottography).
This paper describes an innovative laryngoscopy protocol for mice and rats that permits close-up, unobstructed imaging of breathing and airway protection during swallowing under light anesthesia. The protocol is compatible with a variety of commercially available endoscopy systems in combination with a custom platform to immobilize the anesthetized rodent throughout the procedure. Importantly, numerous designs/configurations of endoscopy platforms are indeed possible, depending on each lab's available resources and research agenda. Our intent here is to provide guidance for researchers to consider in the context of their research. Moreover, we aim to demonstrate how this laryngoscopy protocol can lead to a wealth of objective data that may spark novel insights into our understanding of laryngeal dysfunction and regeneration.
The combined effect of all the steps outlined in this murine laryngoscopy protocol results in a minimally invasive examination of the adult murine larynx that can be repeated in the same animals to detect and characterize laryngeal dysfunction over time in response to iatrogenic injury, disease progression, and/or treatment intervention relative to airway protection. Of note, this protocol does not evaluate vocalization-related laryngeal function.
Protocol
The murine laryngoscopy protocol follows an approved Institutional Animal Care and Use Committee (IACUC) protocol and National Institutes of Health (NIH) Guidelines. It was developed for use with over 100 adult C57BL/6J mice and over 50 adult Sprague Dawley rats, approximately equal sexes and 6 weeks-12 months old for both species. Additional protocol development is necessary for adaptation to younger/smaller rodents. Animals were group housed (up to four mice or two rats per cage, based on sex and litter). The standard vivarium conditions included static caging with strict regulation of ambient temperature (20-26 °C), humidity (30%-70%), and standard 12 h light cycle. All animals received fresh enrichment materials (e.g., hut/pipe, dental treats, nestlet) at weekly cage changes. Unlimited access to food and water was provided, except during a short (up to 4-6 h) food restriction prior to anesthesia as described below. Veterinary and research staff monitored the animals every day.
1. Animal anesthesia that does not abolish swallowing
- Wear appropriate personal protective equipment (e.g., gloves, mask) to minimize allergen exposure while working with rodents.
- Food-restrict each rodent cage up to 4-6 h before anesthesia to minimize the retention of food in the oral cavity and pharynx, which may interfere with endoscopic visualization and/or result in food aspiration during the procedure.
NOTE: Food retention in the oral cavity is a normal finding in rodents without dysphagia if they have not been food-restricted. - Prepare a "warming station" for the induction/recovery of the animals.
- Prewarm a water-circulating heating pad to 37 °C on a benchtop surface.
- Select appropriately sized induction/recovery cages for the species being tested. For example, mouse shoebox cages with filter top lids are appropriately sized for the induction/recovery of mice and rats. Use a fresh induction/recovery cage for each animal being tested; use a single cage as both the induction and recovery cage for the same animal.
- Line the induction/recovery cage floor with a light layer of absorbent material (e.g., aspen shavings, paper towel, puppy pad) for warmth and absorption of body secretions during anesthesia induction and recovery.
- Place the prepared cages (with filter top lids) fully on the heating pad for 30-60 min prior to anesthesia induction.
NOTE: This microenvironment provides sufficient supplemental heat to promote stable anesthesia metabolism during induction and recovery.
- Place the animal's home cage halfway on the preheated 37 °C heating pad for approximately 30 min prior to anesthesia induction.
NOTE: Providing supplemental heat in advance of the procedure may hasten anesthesia induction and prevent accidental overdose due to slowed/delayed anesthesia metabolism from hypothermia. - Prepare ketamine-xylazine (KX) anesthesia based on the species and body weight.
- For mice: A mixture of 90 mg/kg ketamine and 11 mg/kg xylazine is sufficient for transoral laryngoscopy in adult C57BL/6 background mice of either sex. Adjust the doses for other mouse strains and ages.
- For rats: A mixture of 60 mg/kg ketamine and 6 mg/kg xylazine is sufficient for transoral laryngoscopy in adult Sprague Dawley rats of either sex. Adjust the doses for other rat strains and ages.
- Prewarm the syringe-filled anesthetic agents on the 37 °C warming station to prevent heat loss in the animals that occurs when injecting cold fluids.
- Inject the rodent with the calculated KX dose using an appropriately sized syringe (e.g., 1 mL) and needle (e.g., 26 G½).
- For mice: Administer a single subcutaneous (SC) injection.
NOTE: In our experience, SC injections in mice reduce/abolish anesthesia-related mortality compared to intraperitoneal (IP) injections. - For rats: Administer a single SC or IP injection. If preferred, sedate rats with isoflurane (ISO) (3-5%) in an induction chamber immediately prior to KX injection.
NOTE: Spontaneous body movement may resume for a brief period (typically <1 min) until the KX takes effect.
- For mice: Administer a single subcutaneous (SC) injection.
- Administer glycopyrrolate (anticholinergic agent) immediately after the KX injection to reduce excess salivary secretions that may hinder visualization of the larynx during transoral endoscopy and/or mechanically obstruct the upper airway during anesthesia recovery.
NOTE: The dosage and delivery route are identical for mice and rats (0.01-0.02 mg/kg SC), and the effect is nearly immediate and lasts several hours. - After glycopyrrolate injection, place the anesthetized rodent in the preheated induction cage on the warming station and cover the cage with a surgical drape to provide a darkened environment that minimizes visual stimulation for 10 min, undisturbed.
- Terminate the procedure if the rodent remains ambulatory 10 min after the initial KX dose.
NOTE: Attempts to provide additional anesthesia (either KX or ketamine maintenance) will likely be futile.
- Terminate the procedure if the rodent remains ambulatory 10 min after the initial KX dose.
- After 10 min, administer a maintenance dose of ketamine (¼ of the initial dose if hindlimb reflexes are diminished or ½ the initial dose if hindlimb reflexes are brisk; SC for mice and SC/IP for rats) to maintain anesthesia.
- Apply ophthalmic ointment to both eyes to prevent corneal drying and associated trauma during the laryngoscopy procedure.
- Transfer the anesthetized rodent to a custom endoscopy platform to begin the laryngoscopy procedure.
NOTE: We designed our endoscopy platform (Figure 1) to have multiple functionalities for use with a variety of rodent surgical and electrophysiology approaches that do not necessarily require endoscopy. As such, it is overbuilt for purely endoscopic use. Where relevant, we will highlight features/components that are essential to this laryngoscopy protocol. - From this point forward, check hindlimb reflexes every 15-20 min throughout the entire procedure and provide additional ketamine maintenance doses as needed, spaced at least 20 min apart. As this is a relatively short procedure (typically <45 min under anesthesia), additional ketamine is rarely required after the initial maintenance dose.
2. Transoral passage of the endoscope to visualize the larynx
- Prior to anesthetizing the animal, prepare an appropriately sized endoscope with video-recording capability.
NOTE: We routinely use a zero-degree Otoscope with a 1.9 mm shaft diameter and 10 cm shaft length with a custom metal sheath (Figure 2), which is the representative endoscope utilized throughout this protocol.- Connect the endoscope to a light source and endoscopy recording system for real-time viewing and video recording at a minimum of 30 frames per second (fps).
- Focus and white balance the camera for optimal image quality.
- Attach the endoscope to a micromanipulator.
NOTE: For laryngeal motion tracking, we secure the endoscope to a micromanipulator on the endoscopy platform to permit precise endoscope control for stable video capture.
- Secure the rodent in dorsal recumbency on a heated platform. Stabilize and immobilize the head by securing it with ear bars.
- Ensure the rodent's head can freely rotate up/down (but not side-to-side) in the ear bars without slipping out. This degree of freedom facilitates transoral insertion and advancement of the endoscope to reach the larynx.
- If synchronous electrophysiological recording of breathing, swallowing, and swallow-breathing coordination is desired during endoscopy, proceed with applying appropriate sensors for this purpose (Figure 3).
- Secure a respiratory sensor to the abdomen at midline, immediately caudal to the xyphoid process, using surgical tape.
- Shave and clean/disinfect the skin with an alcohol wipe before inserting the needle electrodes to prevent infection.
- Use a 22 G needle to pierce a small opening through the skin before inserting the needle electrode to prevent electromyography (EMG) needle damage.
- Insert a sterile concentric EMG needle electrode (e.g., 25 mm x 0.3 mm/30 G) through the submental skin at midline into the tongue base (e.g., genioglossus or geniohyoid muscle, depending on needle insertion depth).
- Insert a ground electrode (e.g., 27 G stainless steel) subcutaneously at the hip (either side).
- Connect the respiratory sensor and EMG needle electrodes to an electrophysiology recording system (e.g., bioamplifier and data acquisition system with synchronous video capture) and verify clean electrophysiology signals in both channels before proceeding.
- Wrap the electrode connection sites with aluminum foil to shield against electrical noise and improve the signal-to-noise ratio in the corresponding electrophysiological recordings.
- Adjust the respiratory sensor location and EMG needle electrode depth as needed to obtain clean electrophysiology signals in both channels. To follow this protocol, use a 1k sampling rate for respiration and a 20k sampling rate and band pass filter (e.g., 150-3,000 Hz) for EMG.
- Cover the rodent's torso (and respiratory sensor) with a transparent blanket to facilitate thermoregulation while permitting visualization of abdominal motion during respiration. Leave the hindlimbs and lower abdomen freely exposed for access during reflex checks and ketamine maintenance redosing. Ensure the blanket does not restrict abdominal movement during breathing.
- Proceed with transoral endoscopy (Figure 4).
- Open the rodent's mouth by inserting a tapered, cotton-tipped applicator behind the central incisors, perpendicular to the jaw. Rotate the cotton swab on the dorsal surface of the tongue to slightly protrude it from the mouth.
- Using a light finger grip, gently pull the tongue slightly out of the mouth to one side of the central incisors while inserting the endoscope tip into the oral cavity (Figure 4A,B).
- Turn on the light source after inserting the endoscope tip into the mouth to avoid potentially harming the rodent's eyes.
- Insert the endoscope lateral to the incisors on the same side as the retracted tongue. The central incisors prevent the endoscope from being inserted at midline, thus necessitating this lateral insertion approach.
- Start the endoscopy (and electrophysiology) recording systems. Record continuously throughout the entire procedure to ensure sufficient data for post hoc analysis or record at select times, depending on the study's needs.
- Carefully advance the endoscope to visualize the oropharynx, using caution not to scrape against the hard palate or apply excess pressure on the tongue that may cause injury.
- Remove any visible food particles and/or excess salivary secretions using an appropriately sized swab (e.g., a 1.5 mm micro-brush) to minimize aspiration risk as the procedure advances.
- Continue advancing/adjusting the endoscope position until the hypopharynx is centered within the field of view on the monitor and key anatomical structures are identifiable (Figure 4C). At this point, all structures should appear anatomically aligned/symmetric within the camera field of view; otherwise, reposition the endoscope as needed.
- Watch for evoked jaw/tongue movement during endoscope advancement. If absent, proceed without further ketamine redosing. If present, administer a second ketamine maintenance dose (¼ to ½ of the initial dose of ketamine) and wait approximately 5 min to take effect before proceeding. Re-dose only if it has been at least 20 min since the previous injection to avoid oversedation and abolishment of swallowing.
- Examine the rodent's tongue every 5 min throughout the procedure for darkened discoloration, which is indicative of ischemia. To avoid this, reposition the endoscope as needed.
- Apply light pressure to the velum with a micro-probe (e.g., a metal spatula) inserted alongside the endoscope to uncouple the soft palate and epiglottis to visualize the larynx from a distance (Figure 4D). Avoid using the endoscope tip for decoupling, as the applied pressure may cause soft tissue damage or permanently bend/damage the endoscope shaft.
NOTE: Unlike humans, the murine larynx is not directly visible from a transoral perspective. Instead, the epiglottis is mechanically entrapped beneath a mucosal membrane overlying the velum, which results in the formation of a cul-de-sac hypopharyngeal space. Applying light pressure on the velum releases the epiglottis from the velar membrane to give a partial view of the larynx. - Observe for evoked swallows during velum/epiglottis decoupling.
- Identify swallows as abrupt, brief posterior tongue displacements toward the hard palate. This movement typically occurs in synchrony with brief mandibular movement/depression, thus providing a surrogate for the identification of swallowing when the posterior tongue is not readily visible in the endoscope field of view.
- Also identify swallows via tongue EMG bursting activity in conjunction with brief apneic episodes in the electrophysiology recording, both occurring in synchrony with glottic closure events in the endoscopy video.
- In the case of rapid repetitive swallowing indicative of insufficient anesthesia (i.e., too light), re-dose and wait ~5-10 min before proceeding. Wait at least 20 min after the previous ketamine maintenance injection to avoid oversedation and abolishment of swallowing.
- Consider the anesthetic depth to be optimal when only a few swallows are evoked during velar-epiglottic decoupling.
- If swallowing is abolished, anesthesia is too deep for assessing laryngeal airway protection. In this case, wait 5-10 min for ketamine metabolism before proceeding with close-up visualization of the larynx.
3. Close-up, unobstructed video recording of laryngeal motion during breathing and evoked swallowing
NOTE: Synchronous electrophysiological recording of breathing, swallowing, and swallow-breathing coordination is also an option.
- Slowly advance the endoscope between the velum and epiglottis while maintaining the larynx in the center of the field of view (Figure 5A-C).
NOTE: The endoscope tip will readily pass through the velar-epiglottic opening without force. Otherwise, abort the procedure to avoid potential harm to the animal. It is possible to visualize the larynx from a distance, with the endoscope tip in the hypopharynx. However, this approach typically requires manual retraction of the epiglottis, velum, and/or tongue for enhanced visualization of the larynx. However, portions of the larynx typically remain obscured from view, and the retraction devices can restrict laryngeal motion, which may be mistaken as dysfunction. - Continue advancing the endoscope to obtain an unobstructed close-up view of the entire ventral-dorsal and lateral dimensions of the larynx in a single field of view (Figure 5C).
NOTE: The ventral commissure may be obstructed by the epiglottis, particularly in younger/smaller mice. In these cases, attempting to more aggressively manipulate the endoscope tip to visualize the ventral commissure can restrict laryngeal motion, which may be mistaken as dysfunction. It may also block laryngeal airflow resulting in asphyxiation. - Observe the oscillatory motion of the larynx as the rodent inhales (glottal widening) and exhales (glottal narrowing) during each respiratory cycle.
NOTE: The rate and magnitude of laryngeal/glottal motion may vary with anesthetic depth; however, a glottal gap (i.e., air space between the left/right arytenoids and vocal folds) typically remains visible throughout the respiratory cycle in healthy rodents.- If marked glottic narrowing is noted, adjust the endoscope position to ensure unobstructed airflow through the upper airway. For example, avoid applying pressure on the velum, which can cause soft tissue obstruction of the nasal airway. Also avoid inserting the endoscope tip into the glottal space, which can block laryngeal airflow leading to asphyxiation. If in rare cases, breathing ceases, deliver several mid-sternal chest compressions (using one finger) or positive pressure ventilation (using a miniature "resuscitation bag") after removing the endoscope.
- Video record laryngeal respiratory motion for 30-60 s for post hoc evaluation purposes.
- With the larynx still in close-up view, slightly adjust the endoscope tip within the laryngeal inlet to apply mechanical stimulation to the mucosa overlying the velum and/or epiglottis, and evoke swallowing in optimally anesthetized rodents.
- Use micro-level adjustments of the endoscope tip (i.e., ~1 mm in any direction) to prevent mucosal injury and/or airway obstruction.
- Watch for evoked swallows, which can be readily identified as abrupt glottic closure events occurring in synchrony with visible jaw depression, tongue EMG bursting activity, and brief (<1/2 s) apnea that is visible in the respiratory trace.
NOTE: Glottic closure events without co-occurring jaw movement may occur; however, glottic closure is typically incomplete for these cases. We suspect these may be other airway protective reflexes (e.g., laryngeal adductor reflex) emerging as anesthesia is beginning to wear off; however, they are rare/inconsistent occurrences that require further investigation. - Repeat until 5-10 swallows are evoked and video recorded per animal. If swallowing is abolished, remove the endoscope and wait 5-10 min for ketamine metabolism to occur before proceeding.
- Carefully retract, but do not remove, the endoscope into the oropharynx and center the hypopharynx in the field of view to visualize the epiglottis and velum.
- Recouple the velum and epiglottis for resumption of nasal breathing by using a micro-swab to apply light pressure against the tongue base to evoke swallowing and re-entrapment of the epiglottis beneath the velar membrane. If recoupling does not occur within a few attempts, proceed with anesthesia recovery without recoupling to avoid risking laryngeal injury.
- Stop the endoscopy (and electrophysiology) recording.
- Use a saline-soaked cotton swab to moisten the tongue and central incisors and return the tongue to its anatomical position within the oral cavity.
- Detach the ear bars and remove the temperature probe, respiratory sensor, and EMG electrodes from the rodent to proceed with anesthesia recovery.
4. Anesthesia recovery
- Place the animal in a prewarmed recovery cage (i.e., same as the induction cage) on the "warming station" to recover from anesthesia.
- Reapply eye lubricant to prevent drying.
- Administer warmed saline SC for fluid hydration: up to 5 mL for rats and up to 0.5 mL for mice.
- Administer atipamezole SC for xylazine reversal and to increase respiratory drive: 1-2 mg/kg for rats and mice.
- Begin with 2 mg/kg atipamezole, immediately followed by manual stimulation along the rodent's back and stomach to accelerate recovery.
NOTE: Using this approach, spontaneous head movement typically begins within 1-3 min. However, return to ambulatory status typically takes an average of 2 h (ranging from 1 h to 5 h) following laryngoscopy under KX anesthesia, due to individual differences. - Provide additional atipamezole dosed at 1 mg/kg (at least 15 min after the first injection) if spontaneous body movement is diminishing instead of increasing within the first 15-30 min of anesthesia recovery, despite providing frequent manual stimulation.
- Proceed with the administration of doxapram (5 mg/kg IP for rats and mice) if spontaneous activity continues to diminish. Re-dose with this "emergency rescue" agent at 10-15-min intervals (up to five doses) until spontaneous movement emerges. If rodents remain moribund, euthanize the animals using approved euthanasia methods (e.g., an overdose of ketamine followed by a secondary method such as decapitation).
- Begin with 2 mg/kg atipamezole, immediately followed by manual stimulation along the rodent's back and stomach to accelerate recovery.
- Closely monitor recovering rodents at 15-20 min intervals to detect adverse changes in respiratory status, mobility, and thermoregulation and provide intervention as needed (e.g., manual stimulation, supplemental oxygen, thermal blanket, atipamezole, or doxapram injections). Provide more frequent monitoring for rodents requiring intervention.
- Provide supplemental oxygen (e.g., 1-2 L/min in a warmed induction chamber, without ISO) for 10 min intervals as needed for rodents with protracted KX anesthesia recovery times. Alternatively, apply frequent stimulation along the animal's dorsal and ventral surfaces to normalize SpO2 levels (>94%).
- Place recovering cage mates in the same recovery cage (up to two rodents per cage) to promote increased spontaneous activity and faster recovery.
- Return rodents to their warmed home cage when able to spontaneously move around the recovery cage.
- Return the standard food and water bottle to the home cage. Do not provide special accommodations for food/water access while anesthesia is still wearing off to minimize choking/aspiration risk.
- Observe the home cage activity and remove any obstacles that impede ambulation (e.g., hut, PVC pipe).
- Position the home cage half on, half off the warming station for the next 12-16 h (i.e., overnight).
NOTE: Discontinuing supplemental heat earlier may result in mortality due to hypothermia.
- Perform standard health checks the next morning. Return animals with the resumption of normal/baseline activity, body functions (e.g., thermoregulating, eating, drinking, urinating, defecating), and stable weight (i.e., maintaining or gaining) to standard vivarium conditions with routine daily health monitoring. On the rare occurrence that rodents have diminished activity, body functions, or body weight, continue supplemental heat for another day.
NOTE: For rats, it is common for porphyrin staining to suddenly appear around both eyes ~3-6 h into the anesthesia recovery period. The staining typically resolves within 24 h.
5. Objective quantification of laryngeal motion during breathing versus swallowing
- Use video editing software with a frame-by-frame analysis feature to view the endoscopy videos.
- Identify at least one representative 10-20 s episode of spontaneous breathing per animal.
- Identify 3-5 representative swallow events per animal.
- Ensure the selected breathing and swallowing episodes/events meet the following analysis criteria: the larynx centered in the camera field of view with all laryngeal structures/borders visible (i.e., not obscured by the velum, epiglottis, or excess salivary secretions), sufficient lighting (i.e., able to see all laryngeal structures/borders), and without camera motion artifact (i.e., the endoscope is not moving).
- Analyze the identified breathing and swallowing episodes/events using subjective and/or objective approaches.
- For subjective analysis: Use a Likert scale to subjectively score laryngeal motion during real-time and frame-by-frame viewing using video editing software. To follow this protocol, use an expanded Likert scale ranging from -2 to +2, where negative values indicate laryngeal motion in the opposite direction than expected. Estimate laryngeal airway protection during swallowing, where 0 = no reduction in the glottal gap size (i.e., no laryngeal airway protection), 1 = some glottal gap reduction (i.e., incomplete airway protection), and 2 = complete adduction of the arytenoids, with only a small ventral glottal gap between the vocal folds (i.e., complete airway protection), with negative values indicating paradoxical laryngeal motion.
NOTE: A Likert scale ranging from 0 to 2 is commonly used in rodent studies, where 0 = no motion, 1 = some motion, and 2 = normal motion17,18,19,20,21,22. However, this scale does not take into account paradoxical laryngeal motion that often occurs following recurrent laryngeal nerve (RLN) injury10. - For objective analysis: Identify four key video frames – 1) the rest frame directly preceding initiation of laryngeal adduction (i.e., the frame before the vocal folds adduct), 2) the frame in which the vocal folds have completed adduction, 3) the frame immediately preceding abduction of the vocal folds (this may overlap with #2), and 4) the frame where the vocal folds have abducted completely and are returned to a rest position to resume breathing. Use the time stamp of these four key frames to calculate the duration of vocal fold adduction (frame 1 to frame 2), glottic closure (frame 2 to frame 3), vocal fold abduction (frame 3 to frame 4), and total swallow duration (frame 1 to frame 4). Alternatively, use other existing commercial software23 to measure the glottal angle (i.e., between the arytenoids dorsally or the vocal folds ventrally) during maximum abduction and maximum adduction using still-frame images17,18,24. Have at least two trained reviewers perform this process independently in a blinded fashion, identify discrepancies between reviewers, and reach a joint consensus for each discrepancy.
NOTE: We previously performed this manual frame-by-frame analysis of glottic closure timing events (i.e., during airway protective reflexes) using commercial video editing software in rodents and humans14. However, this approach is inefficient and provides only limited quantification of laryngeal motion dynamics. We currently use in-house-built laryngeal tracking software to perform more extensive objective quantification of laryngeal motion during breathing and airway protective reflexes22,25,26,27,28,29,30. The software features automated frame-by-frame tracking capability for objective quantification of left- versus right-sided laryngeal motion distance (amplitude) and timing (frequency). We use these parameters to derive a variety of motion-based measurements to detect/quantify laryngeal dysfunction (e.g., glottal angle maximum/minimum/range, laryngeal motion symmetry, and synchrony) during breathing, swallowing, and other airway protective reflexes (e.g., laryngeal adductor reflex). This software is still undergoing refinement and is not yet commercially/publicly available.
- For subjective analysis: Use a Likert scale to subjectively score laryngeal motion during real-time and frame-by-frame viewing using video editing software. To follow this protocol, use an expanded Likert scale ranging from -2 to +2, where negative values indicate laryngeal motion in the opposite direction than expected. Estimate laryngeal airway protection during swallowing, where 0 = no reduction in the glottal gap size (i.e., no laryngeal airway protection), 1 = some glottal gap reduction (i.e., incomplete airway protection), and 2 = complete adduction of the arytenoids, with only a small ventral glottal gap between the vocal folds (i.e., complete airway protection), with negative values indicating paradoxical laryngeal motion.
Representative Results
Successful use of this murine laryngoscopy protocol results in close-up visualization of the larynx during spontaneous breathing and evoked swallowing under healthy and disease conditions, as shown in Figure 6. Moreover, this protocol can be repeated multiple times in the same rodents to permit investigation of laryngeal function/dysfunction over time. As shown in Figure 7, we successfully repeated this laryngoscopy protocol 6x over a 4-month timespan to investigate the spontaneous recovery pattern in a rat surgical model of RLN injury (data not yet published). Attempts to use ISO anesthesia instead of KX resulted in near abolishment of swallowing (Figure 8) in rodents undergoing direct electrical stimulation of the right superior laryngeal nerve to evoke swallowing, as described in our previous experiments31,32. This occurred with ISO as low as 2%; reducing ISO below this level resulted in the return of spontaneous movement and was therefore avoided. This confounding effect of ISO highlights the importance of anesthesia selection for the successful use of this protocol.
When endoscopic image quality is good, representative video clips of breathing and swallowing can be analyzed using motion tracking software, as shown in Figure 9. Representative outcome measures automatically generated by our custom laryngeal tracking software are listed in Table 1. Note that several breathing- and swallowing-related outcome measures were markedly different between baseline and post-RLN transection in the same representative rat. Whereas glottal angles during breathing were similar between baseline and post-RLN transection, ratios of right/left laryngeal motion amplitude (i.e., mean motion range ratio or MMRR) and frequency (open-close cycle ratio or OCCR) during breathing were lower following transection. Similarly, the swallow duration was shorter following RLN transection.
If synchronous electrophysiological recordings (e.g., respiratory pneumogram and genioglossus EMG) are acquired, several additional objective outcome measures are quantifiable for correlation with laryngoscopy data. Examples of electrophysiology-based outcome measures of interest to our research are summarized in Figure 10. We are currently developing algorithms for automated quantification of these outcome measures.
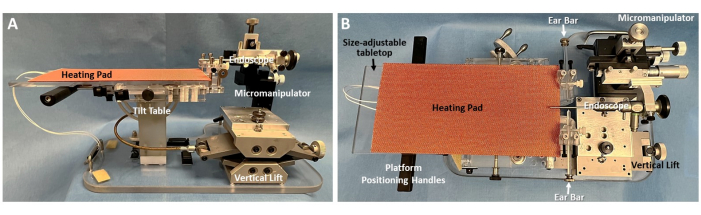
Figure 1: Murine endoscopy platform. (A) Sideand (B) topviews of the custom murine endoscopy platform are shown, with essential components labeled. Note the tabletop beneath the heating pad is size-adjustable. Shown here are the tabletop and heating pad sizes used with rats, which are easily removed to expose a mouse-sized tabletop that accommodates a smaller heating pad (not shown). A custom adapter secures an endoscope to a micromanipulator that is attached to the platform base. This strategic design allows the entire platform to be moved as a unit during the endoscopy procedure, without risking injury to the animal from inadvertent/uncontrolled endoscope motion. The micromanipulator permits gross and micro adjustments of the endoscope tip in multiple directions, including x (left/right), y (forward/back), z (up/down), as well as rotation around y (pitch) and z (yaw). Please click here to view a larger version of this figure.
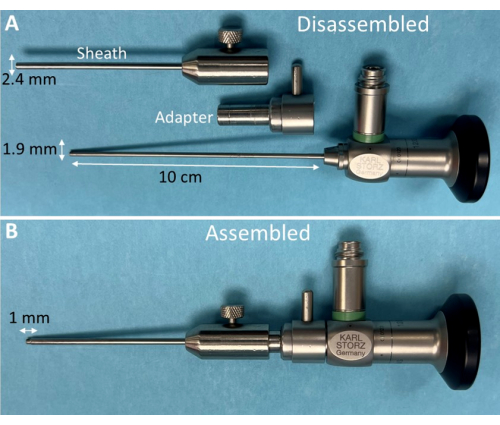
Figure 2: Otoscope and custom sheath for murine laryngoscopy. (A) Disassembled components of a commercial otoscope and custom stainless-steel sheath with adapter for murine laryngoscopy. (B) When assembled, the otoscope tip extends 1 mm beyond the metal sheath but is adjustable up to 5 mm as needed. This strategic design facilitates the advancement of the narrow otoscope tip into the rodent's laryngeal inlet while the slightly larger diameter (2.4 mm) metal sheath sufficiently holds the velum and epiglottis open for optimal visualization of the entire larynx during breathing and swallowing. Please click here to view a larger version of this figure.
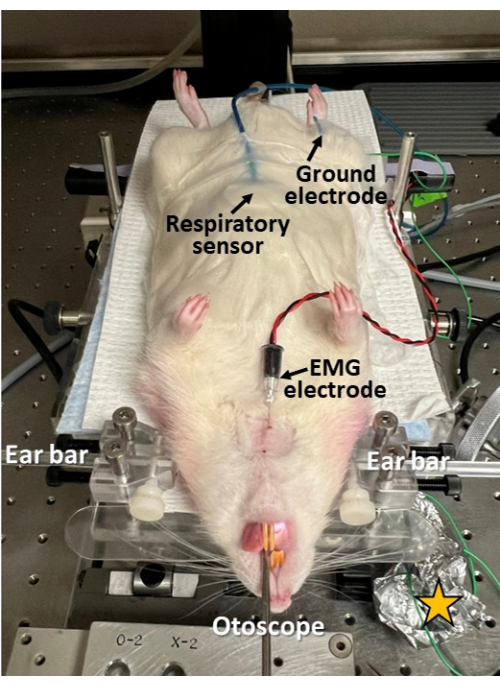
Figure 3: Minimally invasive electrophysiological recording during endoscopy. A respiratory sensor is taped to the rodent's abdomen; an EMG electrode is inserted through the skin into the genioglossus muscle of the tongue; and a ground electrode is inserted subcutaneously at the hip. This approach permits the investigation of swallowing, breathing, and swallow-breathing coordination in synchrony with endoscopy. Note the skin is shaved and cleaned/disinfected at the electrode insertion sites. Yellow star = aluminum foil wrapped around the electrode lead connection sites to improve the signal-to-noise ratio in the electrophysiological recordings. Please click here to view a larger version of this figure.
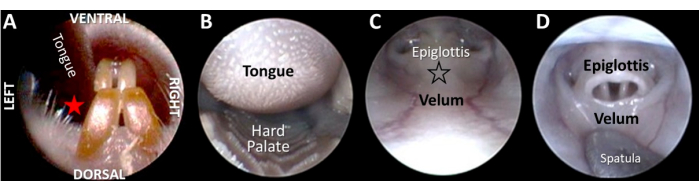
Figure 4: Transoral endoscopy to visualize the larynx from a distance. (A) After gently retracting the tongue with a light finger grip, the endoscope is inserted between the tongue and central incisors at the red star location (i.e., the same side as the retracted tongue to maintain anatomical alignment with the endoscope shaft). (B) As the endoscope is advanced past the hard palate, (C) the epiglottis and velum come into view. (D) To visualize the glottis, the velum and epiglottis must be "decoupled" by applying pressure against the surface of the velum (at the location of the black outlined star in image C). Please click here to view a larger version of this figure.
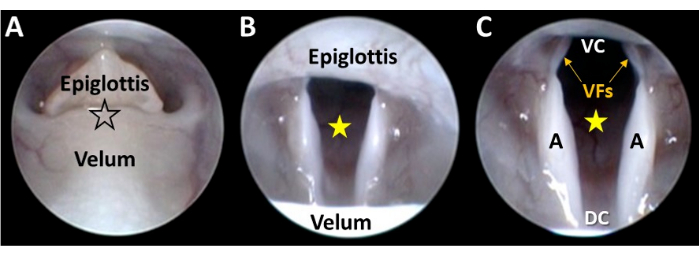
Figure 5: Close-up endoscopic visualization of the larynx. (A) The endoscope tip is gently guided via micromanipulator control between the decoupled velum and epiglottis (at the location of the black-outlined star). As the endoscope advances, (B) the larynx comes into view and the glottal space (yellow star) is centered in the camera field of view via micromanipulator adjustments. (C) Continued micromanipulator advancement of the endoscope results in visualization of the entire ventral-dorsal and lateral dimensions of the larynx. Abbreviations: VC = ventral commissure of the larynx (i.e., the ventral junction point between the vocal folds); DC = dorsal commissure of the larynx (i.e., the dorsal junction point between the arytenoids); VFs = vocal folds; A = arytenoid. Please click here to view a larger version of this figure.
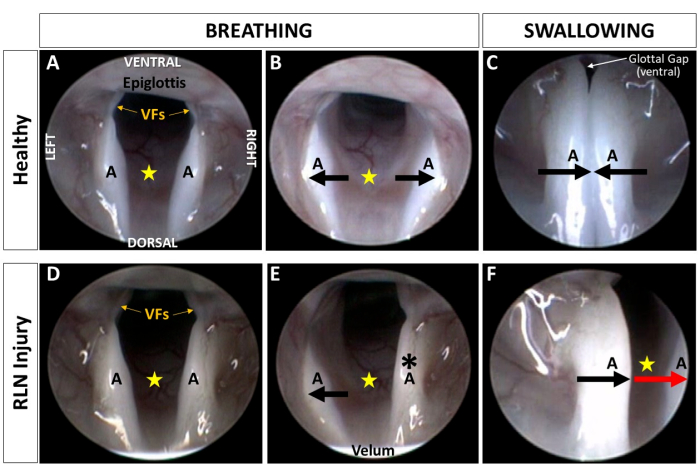
Figure 6: Visualization of the murine larynx during breathing and swallowing. Representative endoscopic images depicting laryngeal motion during breathing and swallowing in an adult Sprague Dawley rat (A–C) before and (D–F) after surgical transection of the right RLN. Note that the resting posture of the larynx appears unchanged (D) following RLN injury compared to (A) baseline. (B,E) During maximum inspiration, laryngeal asymmetry becomes obvious following RLN injury. Instead of both arytenoids abducting to enlarge the glottal space (yellow star), (B) as shown at baseline, (E) the ipsilateral (right) arytenoid (black asterisk) and vocal fold appear immobilized throughout the respiratory cycle following RLN injury. Right-sided asymmetry is also evident during swallowing. (C) At baseline, the arytenoids approximate at midline during swallowing, leaving a small ventral glottal gap between the vocal folds. (F) Following RLN injury, the ipsilateral arytenoid and VF move paradoxically (i.e., in the same direction as the unaffected side, red arrow) during swallowing, leaving a large glottal gap (yellow star) extending from the ventral to posterior laryngeal commissures. (F) This image provides direct evidence of impaired laryngeal airway protection in a rat model of iatrogenic RLN injury. (C,F) Note the larynx moves closer to the endoscope during swallowing, as indicated by the epiglottis and velum no longer being visible in the camera field of view. Black arrows indicate the direction of normal laryngeal motion whereas the red arrow indicates paradoxical motion; yellow star = glottal space. Abbreviations: VFs = vocal folds; A = arytenoid; RLN = recurrent laryngeal nerve. Please click here to view a larger version of this figure.
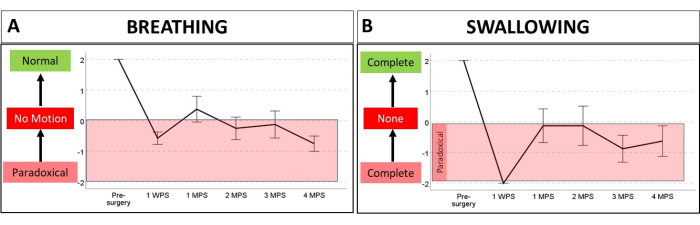
Figure 7: Using serial laryngoscopy to investigate laryngeal dysfunction during breathing and swallowing in a rat model of iatrogenic RLN injury. A Likert scale ranging from -2 to +2 was used to estimate laryngeal motion distance and direction in eight adult Sprague-Dawley rats over a 4 month period. After baseline laryngoscopy, the rats underwent a surgical procedure to transect the right RLN, followed by serial laryngoscopy at 1 week post-surgery, then again at 1 month intervals from 1 to 4 months post-surgery. All eight rats survived the procedure, thus demonstrating the effectiveness of our anesthesia regimen for serial laryngoscopy. (A) Videos were analyzed in real time and frame-by-frame/slow motion to quantify laryngeal motion during breathing, where 0 = no motion, 1 = some motion, and 2 = normal motion distance of the affected (right) side compared to the intact (left) side. (B) For swallowing, the glottal gap size was estimated as follows: 0 = no reduction in the glottal gap size (i.e., no laryngeal airway protection), 1 = some glottal gap reduction (i.e., incomplete airway protection), and 2 = complete adduction of the arytenoids, with only a small ventral glottal gap between the vocal folds (i.e., complete airway protection). Negative values for breathing and swallowing indicate laryngeal motion in the opposite direction than expected (i.e., paradoxical). Note that following RLN injury, both breathing and swallowing were negatively affected. Interestingly, laryngeal airway protection was complete (albeit paradoxical) at the 1 WPS timepoint but worsened thereafter, ranging from no protection to incomplete protection. Abbreviations: WPS = week post-surgery; MPS = months post-surgery; RLN = recurrent laryngeal nerve. Please click here to view a larger version of this figure.
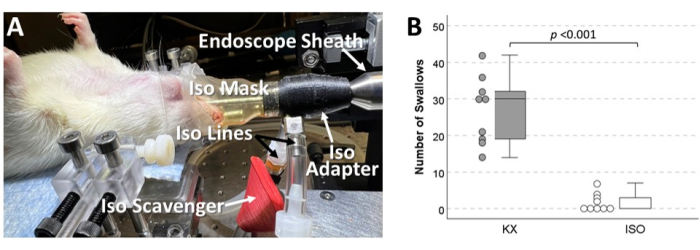
Figure 8: Swallowing inhibited by ISO in rodents. (A) Image of a rodent undergoing laryngoscopy under ISO anesthesia, with labeled components of the custom ISO delivery system designed for this purpose. A major caveat of this innovative approach is the risk of personnel exposure to ISO. (B) Another downside to this approach is ISO suppression of swallowing. This side-by-side boxplot and scatterplot summarizes unpublished data comparing the effect of ISO versus KX anesthesia in mice (9 per group) undergoing direct electrical stimulation of the right superior laryngeal nerve to evoke swallowing. Shown here is the number of swallows evoked during a 5 min trial consisting of 20 s trains of 20 Hz stimulation followed by 10 s of rest. Compared to KX, mice anesthetized with ISO (as low as 2%) had significantly fewer swallows (p < 0.001, independent samples t-test), and swallowing was even abolished in 4/9 mice. Similar findings emerged from non-surgical experiments with both mice and rats (data not shown). Abbreviations: ISO = isoflurane; KX = ketamine-xylazine. Please click here to view a larger version of this figure.
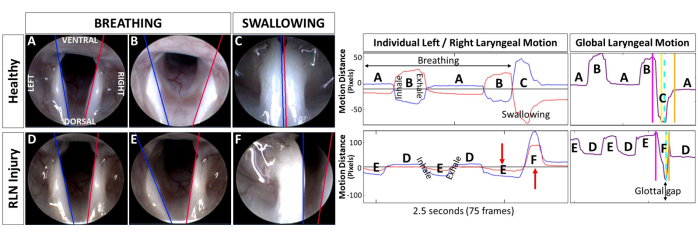
Figure 9: Objective quantification of murine laryngeal motion using tracking software. The same images from Figure 6 showing breathing versus swallowing in a rat at baseline versus post RLN injury are shown here, with laryngeal motion tracking lines added by our custom software. Tracking lines were manually added to the first video frame along the medial border of the arytenoids for automated tracking of left (blue line) versus right (red line) laryngeal motion in the remaining video frames. Corresponding laryngeal motion graphs generated by our custom software from 2.5 s video clips show individual left/right motion versus derived global laryngeal motion, with labels corresponding to (A,D) laryngeal resting posture, (B,E) maximum glottal gap during inspiration, and (C,F) glottic closure during swallowing. Note the paradoxical motion of the right side (red arrows) post RLN injury, as well as the large glottal gap shown in the corresponding derived global motion graph. Representative outcome measures are included in Table 1. Abbreviation: RLN = recurrent laryngeal nerve. Please click here to view a larger version of this figure.
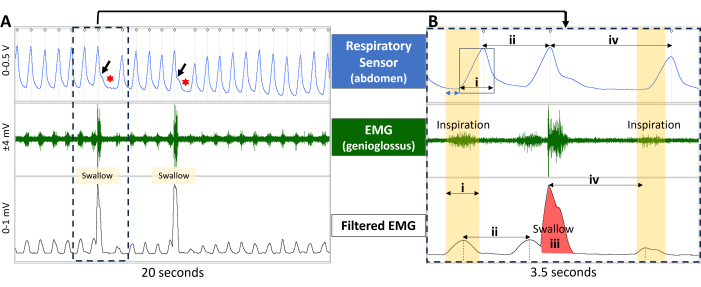
Figure 10: Electrophysiology-based outcome measures for correlation with laryngoscopy data. (A) Electrophysiology recordings during breathing and swallowing are shown for a healthy rat. The top window shows a respiratory trace (from a respiratory sensor taped to the rodent's abdomen), the middle window shows EMG activity in the genioglossus muscle, and the bottom window shows the filtered EMG activity. Note the rhythmic respiratory and EMG pattern during breathing, which is interrupted during swallowing events. Swallow events are readily detected via jagged motion in the respiratory trace (black arrows) that is immediately followed by brief apnea (red asterisk). (B) An expanded window of the dashed rectangular box in A shows how several outcome measures are quantified from the electrophysiological recordings. (A) Note that during inspiration (yellow panels), the respiratory trace (top window) is delayed ~150 ms (blue double arrow) compared to EMG bursting activity, which highlights temporal differences between the two electrophysiological methods. Representative electrophysiology-based outcome measures include 1) inspiratory phase duration (i); 2) inter-respiratory-interval (ii, calculated via the respiratory and filtered EMG channels); swallow area under the curve (iii); and swallow apnea (iv; calculated via the respiratory and filtered EMG channels). Abbreviation: EMG = electromyography. Please click here to view a larger version of this figure.
| Outcome Measures | Baseline | Post-RLN Injury | |
| Breathing | Minimum glottal angle (degrees) | 34.5 | 34.6 |
| Maximum glottal angle (degrees) | 52.9 | 49.9 | |
| Average glottal angle (degrees) | 43.7 | 42.2 | |
| Mean motion range ratio (MMRR) | 1.26 | 0.29 | |
| Open close cycle ratio (OCCR) | 1 | 0.11 | |
| Swallowing | Laryngeal adduction (ms) | 200 | 233 |
| Glottic closure duration (ms) | 67 | 0 | |
| Laryngeal abduction (ms) | 233 | 67 | |
| Total swallow duration (ms) | 500 | 300 | |
Table 1: Representative outcome measures automatically generated by custom laryngeal tracking software. Abbreviation: RLN = recurrent laryngeal nerve.
Supplemental text about the laryngoscopy platform. Please click here to download this File.
Discussion
We have successfully developed a replicable murine-specific laryngoscopy protocol that permits close-up visualization of laryngeal motion during breathing and swallowing. Importantly, the protocol can be performed over time in the same animals to study the impact of various pathological conditions specifically on laryngeal function. This protocol was developed over the past decade and has undergone substantial modification and troubleshooting along the way. Anesthesia optimization was the greatest challenge to overcome to prevent overdosing that abolishes swallowing and/or risks respiratory distress-related mortality. We initially used ISO, which resulted in the abolishment of swallowing, excess saliva production (that obstructs endoscopic visualization), and risk of personnel exposure, which are considered serious contraindications against using ISO for this procedure. We, therefore, focused on KX because it is a commonly used rodent anesthetic33,34,35.
We started our protocol development with mice14,22,29,30,36 while using a sialendoscope because of its smaller shaft diameter (1.1 mm) compared to other potentially suitable endoscopes for this purpose. Importantly, the sialendoscope has a working channel, which we initially used to deliver air pulses to evoke/study the laryngeal adductor reflex14. However, we found the laryngeal adductor reflex was often diminished/abolished in mice and rats, most likely due to general anesthesia and/or inactivation of laryngeal/pharyngeal sensory receptors secondary to mucosal drying from repeated air pulse delivery. Though the laryngeal adductor reflex could not be reliably evoked in our studies, swallowing surprisingly persisted and was readily evoked by mechanical stimulation at/near the laryngeal inlet. For this reason, we switched our focus to endoscopic analysis of mechanically evoked swallowing.
In the process, we abandoned the semi-rigid sialendoscope that was prone to breaking and had insufficient lighting and image resolution to reliably visualize and analyze laryngeal motion. In the exploration of numerous alternative endoscopes, we ultimately settled on a specific otoscope that was suitable for laryngoscopy with both mice and rats. Based on our experience, the most essential feature when selecting a suitable endoscope for murine laryngoscopy is a shaft diameter of less than 2 mm that can transmit sufficiently bright light for high-quality video capture. Larger diameter endoscopes cannot readily pass through the laryngeal inlet in mice and rats for close-up visualization of laryngeal motion. Otoscopes are particularly ideal for this purpose, given their excellent light transmission, rigid/durable design, and relatively low cost compared to other types of endoscopes (e.g., sialendoscope, flexible endoscope). In addition, while manual control of the endoscope is an option in stable hands, we consider micromanipulator control to be an essential feature of this laryngoscopy protocol. Importantly, micromanipulator control of the endoscope allows for stable video recording of laryngeal motion by a single researcher for subsequent quantification. To date, we have successfully used this otoscope-based protocol with adult mice and rats. We suspect smaller diameter endoscope options will be essential to perform laryngoscopy with younger/smaller rodents.
A novel advantage of our laryngoscopy protocol is the ability to visualize airway protection during swallowing in rodents, which is not possible in humans due to epiglottic inversion over the laryngeal inlet that obstructs the glottis from view. Rodents therefore provide a unique opportunity to specifically investigate the mechanisms of normal versus pathological laryngeal airway protection for the ultimate purpose of discovering treatments to effectively restore normal laryngeal function. This unique capability of this murine laryngoscopy protocol is a major advantage over videofluoroscopy (i.e., the other "gold standard" test for dysphagia), which has failed to detect aspiration in the numerous rodent models of dysphagia that we have developed/identified thus far30,36,37,38,39,40. This negative VFSS-based finding can be attributed to several anatomical differences in the upper airway of rodents that are apparent via our transoral endoscopy approach. First, the rodent larynx is positioned high in the nasopharynx where it is concealed by a tightly coupled epiglottis and velum that creates a cul-de-sac oral cavity. Additionally, the epiglottis at rest is entrapped beneath a mucosal sheath overlying the velum. This anatomical configuration results in rodents being obligate nasal breathers; thus, oral breathing in awake rodents is a sign of respiratory morbidity. During swallowing in healthy rodents, however, the epiglottis slides out from the mucosal sheath and inverts over the laryngeal inlet as the larynx elevates further into the nasopharynx, out of the path of the bolus. These dynamic upper airway events can be directly visualized/assessed via laryngoscopy in healthy rodents and models of laryngeal dysfunction.
Importantly, we have shown that despite not aspirating during VFSS testing, rodent models (e.g., iatrogenic RLN injury) indeed show evidence of impaired laryngeal airway protection (i.e., incomplete glottal closure) via laryngoscopy that is translational to human patients with dysphagia-related aspiration. Thus, this murine laryngoscopy protocol provides a useful translational platform to specifically investigate mechanisms of airway protection and targeted treatments, which currently remain elusive. Achieving this goal will require further development/optimization of our current method, which utilizes the endoscope tip to provide uncalibrated mechanical stimulation of the laryngeal/pharyngeal mucosa to evoke swallowing. More rigorous, precisely controlled methods for evoking swallowing are currently being explored in our lab, including direct electrical stimulation of the superior laryngeal nerve32,41 and chemical (e.g., citric acid42) stimulation of the laryngeal/pharyngeal mucosa. An additional limitation of this protocol is the supine positioning of the rodents, which does not mimic awake and natural feeding behavior. Initial protocol development included prone positioning, which resulted in restricted mandibular motion while also limiting visibility of the oral cavity, markedly impeding endoscope passage. It is possible to visualize the larynx from a distance with the endoscope tip in the hypopharynx; however, this approach typically requires manual retraction of the epiglottis, velum, and/or tongue for enhanced visualization of the larynx. We have fabricated a variety of custom manual retraction devices for this purpose (e.g., modified otoscope specula, modified pipette tips). However, portions of the larynx typically remain obscured from view, and the retraction devices can restrict laryngeal motion, which may be mistaken as dysfunction. Moreover, recent additional features of the endoscopy platform (e.g., Trendelenburg tilt, and a cutout between the ear bars to accommodate jaw motion) may facilitate testing rodents in the prone position. Ear bars and supplemental heat are necessary features of the laryngoscopy protocol. Ear bars prevent the head from moving during transoral manipulation of the endoscope. A homeothermic heating system maintains body temperature between 36 °C and 38 °C to promote stable anesthesia and prevent hypothermia throughout the procedure.
Now that methodology exists to reliably video record laryngeal motion during breathing and swallowing in rodents, high-throughput quantification is an essential next step. Therefore, our video analysis efforts are ongoing to determine which outcome measure generated by our custom software can best distinguish healthy from disease conditions as well as detect changes over time in response to natural disease progression or treatment interventions. The top candidates will be the focus of subsequent machine learning approaches to accelerate video imaging analysis. Importantly, cases of suboptimal image quality (e.g., insufficient lighting, anatomical structures outside the field of view, excess secretions obscuring laryngeal structures, etc.) are currently not amenable to laryngeal tracking; however, this barrier may be overcome in the future via machine learning tools. Until then, careful selection of video frame sequences that meet the criteria for laryngeal tracking analysis (as described in protocol section 5 remains paramount.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded in part by two NIH grants: 1) a multi-PI (TL and NN) R01 grant (HL153612) from the National Heart, Lung, and Blood Institute (NHLBI), and 2) an R03 grant (TL, DC0110895) from the National Institute on Deafness and Other Communication Disorders (NIDCD). Our custom laryngeal motion tracking software development was partially funded by a Coulter Foundation grant (TL & Filiz Bunyak). We thank Kate Osman, Chloe Baker, Kennedy Hoelscher, and Zola Stephenson for providing excellent care of our laboratory rodents. We also acknowledge Roderic Schlotzhauer and Cheston Callais from the MU Physics Machine Shop for their design input and fabrication of our custom endoscopy platform and strategic modifications to commercial endoscopes and micromanipulators to meet our research needs. Our custom laryngeal motion tracking software was developed in collaboration with Dr. Filiz Bunyak and Dr. Ali Hamad (MU Electrical Engineering and Computer Science Department). We also thank Jim Marnatti from Karl Storz Endoscopy for providing guidance on otoscope selection. Finally, we would like to recognize numerous previous students/trainees in the Lever Lab whose contributions have informed the development of our current murine laryngoscopy protocol: Marlena Szewczyk, Cameron Hinkel, Abigail Rovnak, Bridget Hopewell, Leslie Shock, Ian Deninger, Chandler Haxton, Murphy Mastin, and Daniel Shu.
Materials
| Atipamezole | Zoetis | Antisedan; 5 mg/mL | Parsippany-Troy Hills, NJ |
| Bioamplifier | Warner Instrument Corp. | DP-304 | Hamden, CT |
| Concentric EMG needle electrode | Chalgren Enterprises, Inc. | 231-025-24TP; 25 mm x 0.3 mm/30 G | Gilroy, CA |
| Cotton tipped applicator (tapered) | Puritan Medical Products | REF 25-826 5W | Guilford, ME |
| Data Acquisition System | ADInstruments | PowerLab 8/30 | Colorado Springs, CO |
| DC Temperature Control System – for endoscopy platform | FHC, Inc. | 40-90-8D | Bowdoin, ME |
| Electrophysiology recording software | ADInstruments | LabChart 8 with video capture module | Colorado Springs, CO |
| Endoscope monitor | Karl Storz Endoscopy-America | Storz Tele Pack X monitor | El Segundo, CA |
| Glycopyrrolate | Piramal Critical Care | NDC 66794-204-02; 0.2 mg/mL | Bethlehem, PA |
| Ground electrode | Consolidated Neuro Supply, Inc. | 27 gauge stainless steel, #S43-438 | Loveland, OH |
| Isoflurane induction chamber | Braintree Scientific, Inc. | Gas Anesthetizing Box – Red | Braintree, MA |
| Ketamine hydrochloride | Covetrus North America | NDC 11695-0703-1, 100 mg/mL | Dublin, OH |
| Metal spatula to decouple epiglottis and velum | Fine Science Tools | Item No. 10091-12; | Foster City, CA |
| Micro-brush to remove food/secretions from oral cavity | Safeco Dental Supply | REF 285-0023, 1.5 mm | Buffalo Grove, IL |
| Mouse-size heating pad for endoscopy platform | FHC, Inc. | 40-90-2-07 – 5 x 12.5 cm Heating Pad | Bowdoin, ME |
| Ophthalmic ointment (sterile) | Allergan, Inc. | Refresh Lacri-lube | Irvine, CA |
| Otoscope | Karl Storz | REF 1232AA | El Segundo, CA |
| Pneumogram Sensor | BIOPAC Systems, Inc. | RX110 | Goleta, CA |
| Pulse oximetry – Vetcorder Pro Veterinary Monitor | Sentier HC, LLC | Part No. 710-1750 | Waukesha, WI |
| Rat-size heating pad for endoscopy platform | FHC, Inc. | 40-90-2 – 12.5X25cm Heating Pad | Bowdoin, ME |
| Sterile needles for drug injections | Becton, Dickinson and Company | REF 305110, 26 G x 3/8 inch, PrecisionGlide | Franklin Lakes, NJ |
| Sterile syringes for drug injections | Becton, Dickinson and Company | REF 309628; 1 mL, Luer-Lok tip | Franklin Lakes, NJ |
| Surgical drape to cover induction cage for dark environment | Covidien LP | Argyle Surgical Drape Material, Single Ply | Minneapolis, MN |
| Surgical tape to secure pneumograph sensor to abdomen | 3M Health Care | #1527-0, 1/2 inch | St. Paul, MN |
| Transparent blanket for thermoregulation | The Glad Products Company | Press’n Seal Cling Film | Oakland, CA |
| Video editing software | Pinnacle Systems, Inc. | Pinnacle Studio, v24 | Mountain View, CA |
| Water circulating heating pad – for anesthesia induction/recovery station | Adroit Medical Systems | HTP-1500 Heat Therapy Pump | Loudon, TN |
| Xylazine | Vet One | NDC 13985-701-10; Anased, 100 mg/mL | Boise, ID |
References
- Brunner, E., Friedrich, G., Kiesler, K., Chibidziura-Priesching, J., Gugatschka, M. Subjective breathing impairment in unilateral vocal fold paralysis. Folia Phoniatr Logop. 63 (3), 142-146 (2011).
- Chandrasekhar, S. S., et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 148 (6 Suppl), S1-S37 (2013).
- Fang, T. J., et al. Quality of life measures and predictors for adults with unilateral vocal cord paralysis. Laryngoscope. 118 (10), 1837-1841 (2008).
- Wang, W., et al. Laryngeal reinnervation using ansa cervicalis for thyroid surgery-related unilateral vocal fold paralysis: a long-term outcome analysis of 237 cases. PLoS One. 6 (4), e19128 (2011).
- Cohen, S. M., et al. Association between dysphagia and inpatient outcomes across frailty level among patients >/= 50 years of age. Dysphagia. 35 (5), 787-797 (2020).
- Poulsen, S. H., et al. Signs of dysphagia and associated outcomes regarding mortality, length of hospital stay and readmissions in acute geriatric patients: Observational prospective study. Clin Nutr ESPEN. 45, 412-419 (2021).
- Lin, R. J., Smith, L. J., Munin, M. C., Sridharan, S., Rosen, C. A. Innervation status in chronic vocal fold paralysis and implications for laryngeal reinnervation. Laryngoscope. 128 (7), 1628-1633 (2018).
- Choi, J. S., et al. Functional regeneration of recurrent laryngeal nerve injury during thyroid surgery using an asymmetrically porous nerve guide conduit in an animal model. Thyroid. 24 (1), 52-59 (2014).
- Wang, B., et al. Neurotrophin expression and laryngeal muscle pathophysiology following recurrent laryngeal nerve transection. Mol Med Rep. 13 (2), 1234-1242 (2016).
- Woodson, G., Randolph, G. W. Pathophysiology of recurrent laryngeal nerve injury. Surgery of the Thyroid and Parathyroid Glands (Third Edition). , 404-409.e2 (2021).
- James, M., Palmer, O. Instrumentation and techniques for examination of the ear, nose, throat, and sinus. Oral Maxillofac Surg Clin North Am. 24 (2), 167-174 (2012).
- Patel, R. R., et al. Recommended protocols for instrumental assessment of voice: American Speech-Language-Hearing Association Expert Panel to develop a protocol for instrumental assessment of vocal function. Am J Speech Lang Pathol. 27 (3), 887-905 (2018).
- Kamarunas, E. E., McCullough, G. H., Guidry, T. J., Mennemeier, M., Schluterman, K. Effects of topical nasal anesthetic on fiberoptic endoscopic examination of swallowing with sensory testing (FEESST). Dysphagia. 29 (1), 33-43 (2014).
- Shock, L. A., et al. Improving the utility of laryngeal adductor reflex testing: a translational tale of mice and men. Otolaryngol Head Neck Surg. 153 (1), 94-101 (2015).
- Aviv, J. E., et al. Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Ann Otol Rhinol Laryngol. 108 (8), 725-730 (1999).
- Farneti, D. The instrumental gold standard: fees. J Gastroenterol Hepatol Res. 3, 1281-1291 (2014).
- Hernandez-Morato, I., et al. Reorganization of laryngeal motoneurons after crush injury in the recurrent laryngeal nerve of the rat. J Anat. 222 (4), 451-461 (2013).
- Hernandez-Morato, I., Sharma, S., Pitman, M. J. Changes in neurotrophic factors of adult rat laryngeal muscles during nerve regeneration. Neurosciences. 333, 44-53 (2016).
- Tessema, B., et al. Evaluation of functional recovery of recurrent laryngeal nerve using transoral laryngeal bipolar electromyography: a rat model. Ann Otol Rhinol Laryngol. 117 (8), 604-608 (2008).
- Tessema, B., et al. Observations of recurrent laryngeal nerve injury and recovery using a rat model. Laryngoscope. 119 (8), 1644-1651 (2009).
- Monaco, G. N., et al. Electrical stimulation and testosterone enhance recovery from recurrent laryngeal nerve crush. Restor Neurol Neurosci. 33 (4), 571-578 (2015).
- Haney, M. M., Hamad, A., Leary, E., Bunyak, F., Lever, T. E. Automated quantification of vocal fold motion in a recurrent laryngeal nerve injury mouse model. Laryngoscope. 129 (7), E247-E254 (2019).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Wang, B., et al. Functional regeneration of the transected recurrent laryngeal nerve using a collagen scaffold loaded with laminin and laminin-binding BDNF and GDNF. Sci Rep. 6, 32292 (2016).
- Hamad, A., Haney, M. M., Lever, T. E., Bunyak, F. Automated segmentation of the vocal folds in laryngeal endoscopy videos using deep convolutional regression networks. , 140-148 (2019).
- Wang, Y. Y., Hamad, A. S., Palaniappan, K., Lever, T. E., Bunyak, F. LARNet-STC: Spatio-temporal orthogonal region selection network for laryngeal closure detection in endoscopy videos. Comput Biol Med. 144, 105339 (2022).
- Lever, T. E., et al. Advancing laryngeal adductor reflex testing beyond sensory threshold detection. Dysphagia. 37 (5), 1151-1171 (2022).
- Wang, Y. Y., Hamad, A. S., Lever, T. E., Bunyak, F. Orthogonal region selection network for laryngeal closure detection in laryngoscopy videos. Annu Int Conf IEEE Eng Med Biol Soc. 2020, 2167-2172 (2020).
- Haney, M. M., et al. Recurrent laryngeal nerve transection in mice results in translational upper airway dysfunction. J Comp Neurol. 528 (4), 574-596 (2020).
- Mok, A., et al. A surgical mouse model for advancing laryngeal nerve regeneration strategies. Dysphagia. 35 (3), 419-437 (2020).
- Haney, M. M., Ericsson, A. C., Lever, T. E. Effects of intraoperative vagal nerve stimulation on the gastrointestinal microbiome in a mouse model of amyotrophic lateral sclerosis. Comp Med. 68 (6), 452-460 (2018).
- Lever, T. E., et al. A mouse model of pharyngeal dysphagia in amyotrophic lateral sclerosis. Dysphagia. 25 (2), 112-126 (2010).
- Struck, M. B., Andrutis, K. A., Ramirez, H. E., Battles, A. H. Effect of a short-term fast on ketamine-xylazine anesthesia in rats. J Am Assoc Lab Anim Sci. 50 (3), 344-348 (2011).
- Richardson, C. A., Flecknell, P. A. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress. Altern Lab Anim. 33 (2), 119-127 (2005).
- Hohlbaum, K., et al. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS One. 13 (9), e0203559 (2018).
- Welby, L., Maynard, T., Zohn, I., Lever, T. Fluoroscopic and endoscopic investigation of dysphagia in a mouse model of DiGeorge syndrome. Dysphagia. 34, 1003-1004 (2019).
- Mueller, M., et al. Impact of limb phenotype on tongue denervation atrophy, dysphagia penetrance, and survival time in a mouse model of ALS. Dysphagia. 37 (6), 1777-1795 (2022).
- Osman, K. L., et al. Optimizing the translational value of mouse models of ALS for dysphagia therapeutic discovery. Dysphagia. 35 (2), 343-359 (2020).
- Lever, T. E., et al. Videofluoroscopic validation of a translational murine model of presbyphagia. Dysphagia. 30, 328-342 (2015).
- Lever, T. E., et al. Adapting human videofluoroscopic swallow study methods to detect and characterize dysphagia in murine disease models. J Vis Exp. (97), 52319 (2015).
- Ballenger, B., et al. Targeted electrical stimulation of the superior laryngeal nerve – a potential treatment for dysphagia in ALS. FASEB J. 36 (S1), (2022).
- Kloepper, A., et al. An experimental swallow evoked potential protocol to investigate the neural substrates of swallowing. OTO Open. 4 (1), (2020).

.
