Time-Lapse Imaging of Migrating Neurons and Glial Progenitors in Embryonic Mouse Brain Slices
Summary
During the development of the cerebral cortex, neurons and glial cells originate in the ventricular zone lining the ventricle and migrate toward the brain surface. Many genes are involved in this process. This protocol introduces the technique for the time-lapse imaging of migrating neurons and glial progenitors.
Abstract
During the development of the cerebral cortex, neurons and glial cells originate in the ventricular zone lining the ventricle and migrate toward the brain surface. This process is crucial for proper brain function, and its dysregulation can result in neurodevelopmental and psychiatric disorders after birth. In fact, many genes responsible for these diseases have been found to be involved in this process, and therefore, revealing how these mutations affect cellular dynamics is important for understanding the pathogenesis of these diseases. This protocol introduces a technique for time-lapse imaging of migrating neurons and glial progenitors in brain slices obtained from mouse embryos. Cells are labeled with fluorescent proteins using in utero electroporation, which visualizes individual cells migrating from the ventricular zone with a high signal-to-noise ratio. Moreover, this in vivo gene transfer system enables us to easily perform gain-of-function or loss-of-function experiments on the given genes by co-electroporation of their expression or knockdown/knockout vectors. Using this protocol, the migratory behavior and migration speed of individual cells, information that is never obtained from fixed brains, can be analyzed.
Introduction
During the development of the cerebral cortex, (apical) radial glia in the pallial ventricular zone (VZ) lining the lateral ventricle produce first neurons and then glial progenitors with some overlapping period1. Neurons are also generated from intermediate progenitors or basal radial glia in the subventricular zone (SVZ) adjacent to the VZ, both of which originate from the (apical) radial glia2,3. In mice, radial glial cells produce only neurons on embryonic day (E) 12-14, both neurons and glial progenitors on E15-16, and glial progenitors from E17 onward4. The major population of glial progenitors generated during these embryonic stages preferentially differentiates into astrocytes, although some cells also differentiate into oligodendrocytes5. Neurons and astrocyte progenitors generated at these stages migrate toward the brain surface and enter the cortical plate (future cortical gray matter). Neuronal migration from the VZ to the cortical plate occurs in multiple phases. Neurons first adopt a multipolar morphology just above the multipolar cell accumulation zone (MAZ), overlapping the SVZ or intermediate zone, where they vigorously extend and retract multiple thin processes and slowly migrate (multipolar migration)6,7. After approximately 24 h, neurons transform into a bipolar morphology, extending a thick leading process toward the brain surface and a thin trailing process backward, and migrate linearly toward the brain surface using a radial process extending from the radial glia to the pial surface as a scaffold, which is called locomotion mode2,8. Because neurons in locomotion mode always reach the outermost surface of the cortical plate, passing through their predecessors just under the marginal zone, neurons are aligned in a birthdate-dependent inside-out manner in the cortical plate9,10,11.
In contrast, astrocyte progenitors migrate rapidly to the intermediate zone and cortical plate, with frequent directional changes. This migratory behavior is completely different from neuronal migration and is called erratic migration5. Astrocyte progenitors also migrate along blood vessels in a process called blood vessel-guided migration. Astrocyte progenitors switch between these migration modes and reach the cortical plate5,12. Although the positioning of astrocytes is not strictly determined by their date of production, a mild tendency for early-born astrocytes to settle in the superficial part of the cortical plate has been observed5. Interestingly, astrocytes that settle in the cortical plate are generated in embryonic stages and eventually differentiate into protoplasmic astrocytes, whereas postnatally generated astrocytes do not migrate actively, remain in the white matter, and differentiate into fibrous astrocytes5. How this stage-dependent specification of astrocytic subtypes occurs remains unclear.
A growing number of genes involved in neuronal migration have been identified, including those involved in neurodevelopmental and psychiatric disorders13,14. Therefore, it is crucial to elucidate the effects of mutations in these genes on the behavior of migrating neurons. As previously mentioned, neuronal migration occurs in multiple phases. Time-lapse observations can directly determine the phase that is mainly affected (cell cycle exit, multipolar-bipolar transition, migration speed of locomotion, etc.). However, the molecular mechanisms underlying the specification, migration, and positioning of astrocytes remain largely unknown. Given that astrocytes play crucial roles in synaptogenesis15 and blood-brain barrier formation during brain development16, developmental defects in astrocytes may result in neurodevelopmental disorders. Time-lapse studies on astrocyte progenitors may clarify these molecular mechanisms and their relationship with mental illness.
This protocol provides a method for time-lapse observation of cortical VZ-derived cells. A similar video protocol for the observation of neuronal migration has already been published17. Here, we describe the method for both migrating neurons and astrocyte progenitors. To label these cells with fluorescent proteins, such as green and red fluorescent proteins (GFP and RFP), plasmid mixtures containing appropriate components are introduced into the cortical VZ by in utero electroporation at appropriate stages18,19,20,21. The manipulated embryos are removed at the desired stages, and the brains are sliced and used for time-lapse observations using a laser scanning microscope. The migration speed, direction, and other behaviors, which are never addressed using fixed brain samples, can be examined using this method. Using in utero electroporation, expression, and knockdown/knockout vectors can be easily transferred concomitantly with fluorescent protein vectors, enabling us to conduct gain-of-function and loss-of-function studies of specific genes.
Protocol
The present study was performed with the approval of and following the guidelines of the Animal Care and Use Committee of the Institute for Developmental Research, Aichi Developmental Disability Center (#2019-013), and Keio University (A2021-030). Timed pregnant ICR (wild-type) mice were obtained commercially (see Table of Materials). To observe the relationship between migrating cells and blood vessels, Flt1-DsRed mice, in which the endothelial cells express DsRed22, were used. Male Flt1-DsRed mice were crossed with female ICR mice (both mice were at the age of 8-24 weeks). All the animals were housed and manipulated under specific pathogen-free (SPF) conditions until sampling. The reagents and equipment used in this study are listed in the Table of Materials.
1. In utero electroporation
- Prepare HEPES-buffered saline (HBS) solution by adding an equal volume of autoclaved water to commercially available 2x HBS.
- There are two options of anesthetics; isoflurane (inhalation) and MMB (injection). In the case of using MMB, prepare it as follows. Add 0.75 mL of medetomidine (1 mg/mL), 2 mL of midazolam (5 mg/mL), and 2.5 mL of butorphanol (5 mg/mL) in 19.75 mL of autoclaved water23.
NOTE: This anesthetic solution becomes less active within 2 months. Store at 4 °C with light-shield. - Prepare atipamezole solution. Dilute 0.75 mL of atipamezole (5 mg/mL) with 24.25 mL of autoclaved water.
- Prepare 0.2% (w/v) Fast Green solution in autoclaved water. Sterilize with a filter (pore size 0.22 µm).
- Prepare plasmid using a conventional plasmid purification kit based on alkaline lysis and column purification23 according to the manufacturer's instructions (see Table of Materials). Dissolve the purified plasmid from 100 mL bacteria culture in 30-50 µL of HBS to prepare the plasmid stock (3-5 µg/µL) (Supplementary File 1).
- Mix and dilute the plasmid stocks with HBS, and add 1/10 volume of 0.2% Fast Green to color the injectant. The components and final concentrations of the plasmids are determined according to their purpose (Table 1).
- Make the micropipettes by pulling the glass capillaries using a pipette puller.
NOTE: The tip of the pipette is cut obliquely using fine forceps. The outer diameter of the tip is approximately 70 µm. Mark the micropipettes at 2 µL intervals by sucking 2 µL water. - Inject MMB anesthetic at a dose of 10 μL/g body weight intraperitoneally into a pregnant mouse at an appropriate stage (following institutionally approved protocols).
NOTE: The embryonic stages to be manipulated should be determined according to the purpose. To label future II/III pyramidal neurons, E14.5 is suitable. To label glial progenitors, electroporation must be performed on E15-16. Alternatively, the mice can be anesthetized by inhalation of isoflurane (1%-2%). - After the mouse is deeply anesthetized, place it on a warm plate at 38 °C.
NOTE: Ensure that the mouse does not respond to pinching the tail. - After removing fur around the surgical site using depilatory cream, disinfect the surgical area several times in a circular motion with both an iodine-based scrub and 70% alcohol. Make a 2 cm long skin incision along the midline from the level of the 2nd most posterior pair of nipples anteriorly, followed by a 2 cm midline incision in the abdominal wall along the linea alba (Figure 1A).
NOTE: In the process of incision in the abdominal wall, lift it slightly with fine forceps so as not to cut the internal organs. - Place a sterile plastic sheet on the skin and a piece of sterile tissue paper on it (Figure 1A). Create a hole in the center to expose the surgical area.
NOTE: The sterile plastic sheet and the sterile tissue paper are placed after making the incision because a wide view field is required to position the incision referring to the position of the nipples. - Wet the paper with sterile PBS, carefully observe the uterine horns from the incision, and draw out the uterine horn through the holes of the plastic sheet and paper.
- Using a micropipette attached to an aspirator tube assembly, inject 1 µL of the plasmid mix into the left or right lateral ventricle through the uterine wall by expiratory pressure (Figure 1A).
NOTE: Alternatively, plasmid can be injected by using a microinjector. - Pinch the head of the embryo with a tweezer-type electrode and apply electronic pulses using an electroporator (35 V for E14-16, 50 V for E17, 50 ms square pulses with 450 ms intervals, 5 times) (Figure 1A).
NOTE: Do not pinch the head of the embryos strongly, which will damage the embryos. The actual current should be 40-70 mA. - Place the uterine horn back into the abdominal cavity.
- Suture the abdominal wall using a 5-0 silk thread.
- Apply atipamezole solution at a dose of 10 µL/g body weight intraperitoneally.
- Suture the skin using a 5-0 silk thread.
NOTE: The skin can be closed by using an autoclip. - Keep the manipulated mice on a warm plate for at least 30 min. The mice will start moving in the cage.
NOTE: The procedure of in utero electroporation (from steps 1.7 to 1.16) should be done within 30 min. The typical operation time is 20 min.
2. Preparation of slices
NOTE: The freshness of the slices is the key to the success of this experiment. The slices must be prepared (from steps 2.5-2.12) within 2 h.
- Prepare the Hanks' balanced salt solution with Ca2+ and Mg2+; HBSS(+). Add 5 mL of CaCl2 (14 g/L, autoclaved) and 5 mL of MgSO4.7H2O (20 g/L, autoclaved) to 500 mL of HBSS(-). Store at 4 °C. Bubble the solution with 95% O2 + 5% CO2 gas for 5 min on ice before use.
- Prepare the culture medium. At least 2.5 mL/dish is needed. Add 2 mL of fetal bovine serum, 25 µL of L-glutamine (200 mM), 200 µL B27 (50x), 50 µL of penicillin + streptomycin (10 K units/mL and 10 mg/mL) to 8 mL of Neurobasal medium.
- Prepare 3% low melting temperature agarose gel. Pore 50 mL of HBSS(+), then add 1.5 mg of low melting temperature agarose, and autoclave it. Melt the agarose in a microwave oven and keep it at 50 °C in a water bath incubator prior to use.
- Add 1.9 mL of culture medium to a glass base dish. Put a cell culture insert on it carefully to not trap air bulbs under the filter, then add 500 µL of culture medium onto the filter (Figure 1B). Place it on ice.
- After anesthetizing the manipulated mice by inhalation of 5% isoflurane, sacrifice them by cervical dislocation (following institutionally approved protocols) at appropriate stages to be observed.
NOTE: Two days (48 h) and 3 days after electroporation at E14.5 are suitable for observing the multipolar-bipolar transition and locomotion mode, respectively. - Take out the embryos and place them in a Petri dish with cold PBS.
- Select the embryos by the expression of GFP or other fluorescent proteins under a microscope.
- After decapitation of the embryos, dissect out the brains6 under a dissecting microscope and keep them in cold HBSS(+).
- Pour the melted 3% low melting temperature agarose gel into a cryomold (see Table of Materials), and then put the brains in it and roll them several times. Place it at room temperature until the agarose completely solidifies, and then place it on ice.
- Slice the brains coronally at the thickness of 350 µm using a vibrating microtome (see Table of Materials).
- Arrange the slices on the filter (maximum 6 slices per filter).
- Withdraw the culture medium from the inside and outside of the filter cup (600 µL in total). In this step, the slices are pressed and fixed onto a filter. After 5 min, add 100 µL of culture medium to the inside of the cell culture insert.
3. Time-lapse imaging
- Keep the culture chamber mounted on the stage of an inverted laser scan microscope at 37 °C using the heater of the chamber or a hood enclosing the microscope at least 30 min before imaging to prevent Z drift. Supply humidified 5% CO2 + 40% O2 gas to the culture chamber.
- Set the culture dish with slices prepared above in the culture chamber.
- Using a long working distance 20x lens (NA = 0.45), acquire around 10 Z-stack images with 5 µm steps every 15-30 min for 24 h.
NOTE: The use of a motorized XY stage is highly recommended. This enables us to obtain images from multiple objective fields. Typically, we obtain 10-20 images from different objective fields in one time-lapse experiment.
4. Imaging analysis
- Convert XYZT images to XYT by the maximum projection of the Z axis using ImageJ or other software.
NOTE: If the slices slid during the observation, adjust the position of the cells within the images using StackRed, an ImageJ plugin, with its function of Rigid Body transformation (see Table of Materials). - Trace the trajectory of GFP- or other fluorescent protein-positive cells either manually or automatically. Manual and automatic tracing can be performed using MTrackJ24 and TrackMate25,26 (ImageJ plugins), respectively. Automatic tracing using TrackMate is efficient for analyzing many cells in an unbiased manner. However, manual tracing using MTrackJ remains useful for the accurate tracking of individual cells.
Representative Results
Radial glial cells in the pallial VZ produce only neurons until E14, and both neurons and glial cells at E15 and E16. To observe the migratory behaviors of neurons and glial cells simultaneously, we labeled them with enhanced GFP (EGFP) and RFP, respectively, by using a neuron-specific promoter, Tα1 promoter27, and human glial fibrillary acidic protein (hGFAP) promoter28, which is preferentially activated in astrocytes. Astrocyte progenitors repeat cell divisions during the migration, leading to the dilution of plasmid in the cells. To avoid this, a transposon vector system consisting of a donor plasmid (pPB) and a transposase expression vector (pCAG-hyPBase) was used29,30. The donor plasmid used here (pPB-CAG-LNL-RFP) has loxP-neo-loxP (LNL) cassette, and expresses RFP depending on Cre recombinase. ICR mice (wild type; WT) were electroporated with the indicated plasmids in Figure 2A at E15, and the slices were prepared 3 days later. During this period of time, the first cohort of EGFP+ neurons reached the surface of the cortical plate (Figure 2B), but many EGFP+ neurons were still migrating. Time-lapse observation was performed for 24 h. During this observation, neurons with a thick leading process migrated linearly toward the brain surface at a relatively constant rate in the cortical plate (19.54 ± 6.21 µm/h, mean ± standard deviation, 70 cells). The migration trajectories were traced automatically by using TrackMate software (magenta lines in Figure 2B). In the same objective field, astrocyte progenitors were visualized with RFP (Figure 2C). Because the hGFAP promoter is also active in radial glial cells in late embryonic stages, some neurons derived from those radial glia also express RFP (blue arrows in Figure 2B,C). These neurons are almost always transfected with an EGFP expression vector. Therefore, astrocyte progenitors were identified as RFP single-positive cells.
In contrast to neurons, astrocyte progenitors migrate rapidly and randomly with frequent directional changes within the intermediate zone and cortical plate (erratic migration)5. Migration of astrocyte progenitors was traced manually by MTrackJ software (magenta lines in Figure 2C), which revealed their high motility (88.37 ± 33.61 µm/h, 5 cells). To observe the relationship between astrocyte progenitors and blood vessels, Flt1-DsRed mice, which express DsRed, an RFP, in endothelial cells, were used. In this experiment, the astrocyte progenitors were supposed to be identified only by EGFP. To this end, the rDIO system5 was developed, in which EGFP expression depending on hGFAP-Cre can be eliminated in neurons by using a Dcx promoter (an early neuron-specific promoter)31-dependent activation of another set of DNA recombinase and its recognition sequence (Dre-rox) (Figure 2D, the sequence of pPB-CAG-rDIO-EGFP is provided in Supplementary File 1)32,33. The rDIO system was introduced into Flt1-DsRed mouse at E15 by in utero electroporation, and slices were prepared 24 h later. As a result, it was observed that the EGFP-positive astrocyte progenitors actively migrated along blood vessels (blood vessel-guided migration)5 (Figure 2E).
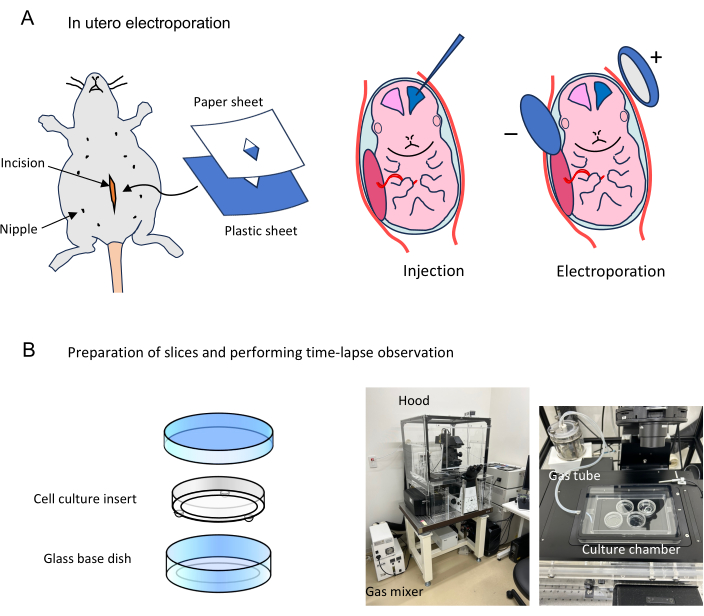
Figure 1: Flow of time-lapse observations of cells labeled by in utero electroporation. (A) A pregnant mouse at an appropriate stage is anesthetized, and the abdominal cavity is cut open. The surgical area is covered with sterile plastic and sterile paper sheets. The uterine horns are exposed, and embryos are injected with 1 µL of plasmid mixture and applied electronic pulses. The abdominal wall and skin are sutured, and the manipulated mice are placed back in the home cage. (B) Slices prepared from electroporated brains are set on the filter of cell culture insert (6 slices maximum) in a glass base dish. The glass base dishes containing slices are mounted in a culture chamber. This time-lapse system has an acryl hood covering the whole microscope to keep the temperature at 37 °C. The culture chamber, in which humidified mixed gas is supplied, is equipped with a microscope stage inside the hood. Please click here to view a larger version of this figure.
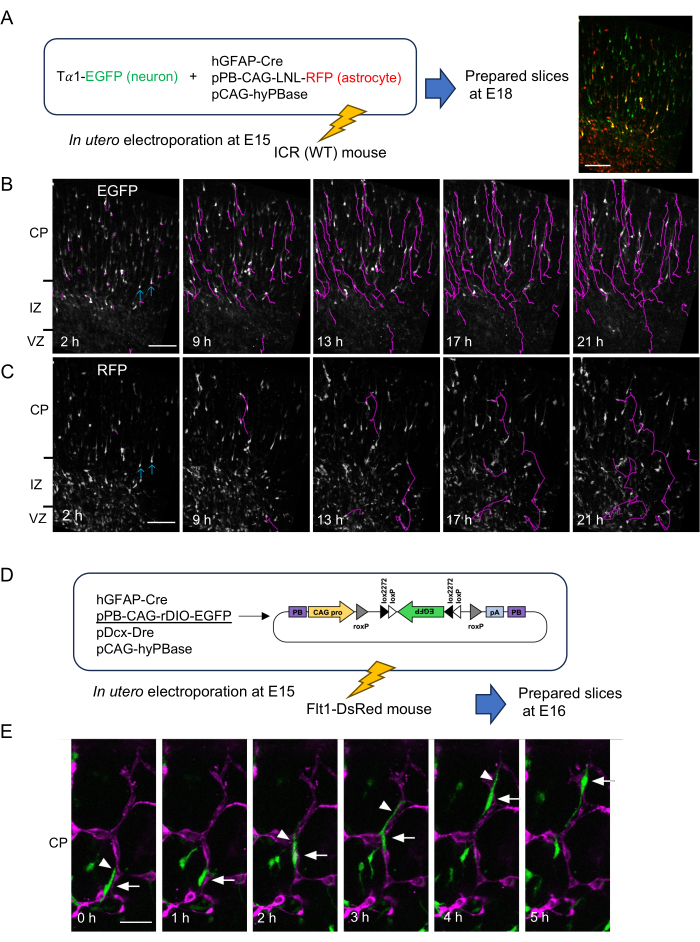
Figure 2: Time-lapse observations of neurons and astrocyte progenitors. (A) Method for differential labeling of neurons (EGFP, green) and astrocyte progenitors (RFP, red), and a representative image of a slice prepared 2 days after the electroporation at 2 h from the start of time-lapse imaging (right). (B,C) Time-lapse images of EGFP+ (B) and RFP+ cells (C) on the same objective field shown in (A)-right from 2 h to 21 h of observation. (B) Trajectories of EGFP+ migrating cells detected automatically by TrackMate software were shown in magenta. (C) Trajectories of RFP+ cells were traced manually using MTrackJ software. EGFP/RFP double positive cells, which are thought to be neurons, are indicated by blue arrows. (D) Labeling strategy of astrocyte progenitors using rDIO system in Flt1-DsRed mouse embryos. (E) Representative result of blood vessel-guided migration. A cell (arrow) protruded a leading process (arrowhead), and migrated along the DsRed-expressing blood vessel (magenta) toward the pial surface. CP; cortical plate. IZ; intermediate zone. VZ; ventricular zone. Scale bars: 200 µm (A–C), 50 µm (E). This figure is adapted from Tabata H. et al.5. Please click here to view a larger version of this figure.
| Neurons (layers II/III) | Astrocyte progenitors | rDIO system (astrocyte specific labeling) |
|||
| pCAG-EGFP | 0.5 µg/µL | pPB-CAG-LNL-EGFP | 0.5 µg/µL | pPB-CAG-rDIO-EGFP | 0.5 µg/µL |
| or Ta1-EGFP (more specific for neurons) | 0.5 µg/µL | hGFAP-Cre | 0.75 µg/µL | hGFAP-Cre | 0.75 µg/µL |
| pCAG-hyPBase | 0.5 µg/µL | pDcx-Dre | 0.5 µg/µL | ||
| pCAG-hyPBase | 0.5 µg/µL | ||||
Table 1: Representative components of plasmid mix for each target. EGFP vectors can be replaced with equivalent RFP or other fluorescent protein expression vectors. To label astrocyte progenitors, a transposon vector system is used. pPB vectors are PiggyBac transposon vectors for genome integration. pCAG-hyPBase is a PiggyBac transposase expression vector. LNL is a loxP-neo-loxP cassette for Cre-dependent expression. rDIO-EGFP is Cre-loxP and Dre-roxP dependent on/off cassette of EGFP expression. To observe the gain-of-function or loss-of-function phenotype of desired genes, expression vectors or knockdown vectors can be added at 1 µg/µL. The concentration of these vectors should be adjusted in each case.
Supplementary File 1: Plasmid sequences. Please click here to download this File.
Discussion
This protocol introduced a method for the time-lapse observation of cells derived from the pallial (cortical) VZ. To label the migrating cells from the VZ, we used in utero electroporation, in which individual cells were clearly labeled with a higher signal-to-noise ratio than in viral vector-mediated labeling. Using in utero electroporation, any type of vector in any combination can be easily introduced into the radial glial cells (neural stem cells) in living embryos. Neurons and glial progenitors can be selectively labeled using different promoters. Since the copy number of plasmids introduced by in utero electroporation is high, gain-of-function and loss-of-function studies can be easily conducted by co-electroporation of GFP expression vectors and various vectors related to the genes of interest, such as expression vectors and knockdown/knockout vectors. Moreover, in utero electroporation can be used to introduce plasmid vectors into the VZ of various brain regions other than the cortical VZ, which enables us to observe the migration of other cell types such as inhibitory neurons, oligodendrocyte progenitors from ganglionic eminences34,35,36 and hippocampal pyramidal neurons37.
In time-lapse studies, the effects of gain-of-function and loss-of-function experiments on cell behavior can be evaluated using the TrackMate and MTrackJ software. Many genes, including those responsible for neurodevelopmental disorders and intellectual disabilities, are involved in neuronal migration. Time-lapse studies can directly reveal how these genes regulate cell behavior. Compared with neuronal migration, the molecular mechanisms of glial cell migration are not well understood. This method may reveal not only the normal development of glial cells, but also the genetic and environmental factors that can affect glial development and its relationship with mental illness.
The limitation of this method is that it inevitably depends on the in vitro slice culture method. Interestingly, migrating neurons do not lose directional cues for migration toward the brain surface, even in slices cultivated in vitro. However, investigators must be careful about artifacts. Cells near the surface are attached to the filter, and the edges of the slices are easily deformed. These cells should be excluded from the analysis. Control experiments should be considered if possible. It is important to note that brain slices recapitulate normal development within approximately 24 h. Therefore, the timing of slice preparation from electroporation should be determined so that the events to be analyzed will occur in this time window. To observe the multipolar-bipolar transition, slices should be prepared 2 days after electroporation. To analyze migration in the locomotion mode, 3 days later is appropriate. The observation of blood vessel-guided migration is a more difficult task than that of other experiments. The endothelial cells are easily degraded in slices without blood flow. To minimize blood vessel degradation, the slices should be prepared as quickly as possible and maintained in a medium containing 20% serum and 40% O2 gas. Other complementary experiments, such as histological analyses of fixed brains or in vivo live imaging using two-photon microscopy, may strengthen the observations found in slice time-lapse analyses.
In summary, this method is the only option for observing cell behavior for 24 h. With the development of novel plasmid vectors, the range of applications of this method will expand further in the future.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Tα1 promoter is a gift from P. Barker and F.D. Miller. Dcx promoter is a gift from Q. Lu. hGFAP-Cre was a gift from Albee Messing. The PiggyBac transposon vector system was provided by the Sanger Institute. Flt1-DsRed mice were provided by M. Ema (Shiga University). This work was supported by JSPS KAKENHI (Grant Number JP21K07309 to H. Tabata, JP20H05688 and JP22K19365 to K. Nakajima) and Takeda Science Foundation, Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research, Keio Gijuku Academic Development Funds to K. Nakajima.
Materials
| Aspirator tube assembly | Drummond | 2-040-000 | |
| Atipamezole (5 mg/mL) | Meiji | Mepatia | |
| Autoclip | Becton Dickinson | 427630 | 9 mm |
| B27 supplement | Gibco | 17504-044 | |
| Butorphanol (5 mg/mL) | Meiji | Vetorphale | |
| Cell culture insert | Millipore | PICM ORG 50 | |
| Confocal microscope | Nikon | A1RHD25 | Equipped with a long working distance lens (S Plan Fluor ELWD 20XC) |
| Cryomold | Tissue-Tek | 4566 | |
| Culture chamber | Tokken | TK-NBCMP | Custom-made |
| Electroporator | NEPA Gene | NEPA21 | |
| Fast Green | Sigma-Aldrich | F7258 | |
| Gas mixer | Tokken | TK-MIGM01-02 | |
| Glass base dish | Iwaki | 3910-035 | Diameter of glass base is 27 mm |
| Glass capillaries | Narishige | GD-1 | |
| HBS (2x) | Sigma-Aldrich | 51558 | |
| HBSS(-) | Wako | 084-08345 | |
| Heater Unit | Tokken | TK-0003HU20 | Custom-made, including hood and heater |
| hGFAP-Cre | Addgene | #40591 | A gift from Albee Messing |
| ImageJ | https://imagej.net/ij/ | ||
| L-glutamine (200 mM) | Gibco | 25030 | |
| Low melting temperature agarose | Lonza | 50100 | |
| Medetomidine (1 mg/mL) | Meiji | Medetomin | |
| Microinjector | Narishige | IM-300 | |
| Midazolam (5 mg/mL) | Sandoz | Midazolam | |
| MTrackJ | https://imagescience.org/meijering/software/mtrackj/ | ||
| Neurobasal medium | Gibco | 21103-049 | |
| pCAG-hyPBase | The hyPBase cDNA from pCMV-hyPBase (a gift from Sanger Institute) was inserted into the downstream of the CAG promoter of pCAGGS (a gift from J. Miyazaki). | ||
| pDcx-Dre | The Dcx promoter from Dcx4kbEGFP70 (a gift from Q. Lu) was exchanged with CAG promoter of pCAG-NLS-HA-Dre34 (a gift from Pawel Pelczar, Addgene #51272). | ||
| Penicillin + Streptomycin | Gibco | 15140122 | |
| Plasmid purification kit | Invitrogen | PureLink HiPure plasmid midiprep kit (K210005) | |
| pPB-CAG-LNL-RFP | CAG-LNL cassette from pCALNL-DsRed (a gift from Connie Cepko, Addgene #13769), and TurboRFP cDNA (Evrogen, FP232) were inserted into the cloning site of pPB-CAG.EBNXN (a gift from Sanger Institute). | ||
| pPB-CAG-rDIO-EGFP | The sequence containning synthetic rox sites, synthetic DIO cassette, and EGFP cDNA from pEGFP-N1 (Clontech, U55762) in reverse direction were inserted into the cloning site of pPB-CAG.EBNXN (a gift from Sanger Institute). The sequence is provided in the Supplementary File. | ||
| Puller | Narishige | PN-31 | |
| StackRed | a plugin for ImageJ | http://bigwww.epfl.ch/thevenaz/stackreg/ | |
| Suture needle | Nazme | C-24-521-R No.1 | 1/2 circle, length 14 mm |
| Suture thread | Nazme | C-23-B2 | Silk, size 5-0 |
| Timed pregnant ICR (wild-type) mice | Japan SLC | ICR mouse | |
| TrackMate | https://imagej.net/plugins/trackmate/index | ||
| Tweezer-type electrode | BEX or NEPA Gene | CUY650P5 | |
| Tα1-EGFP | EGFP cDNA from pEGFP-N1 (Clontech, U55762) was inserted into the downstream of the Tα1 promoter in plasmid 253 (a gift from P. Barker and F.D.Miller) | ||
| Vibrating microtome | Leica or Zeiss | Vibrating blade microtome VT1000S or Hyrax V50. |
References
- Kriegstein, A., Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 32 (1), 149-184 (2009).
- Noctor, S. C., Martínez-Cerdeño, V., Ivic, L., Kriegstein, A. R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 7 (2), 136-144 (2004).
- Haubensak, W., Attardo, A., Denk, W., Huttner, W. B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: A major site of neurogenesis. P Natl Acad Sci USA. 101 (9), 3196-3201 (2004).
- Yoshida, A., Yamaguchi, Y., Nonomura, K., Kawakami, K., Takahashi, Y., Miura, M. Simultaneous expression of different transgenes in neurons and glia by combining in utero electroporation with the Tol2 transposon-mediated gene transfer system. Genes Cells. 15 (5), 501-512 (2010).
- Tabata, H., et al. Erratic and blood vessel-guided migration of astrocyte progenitors in the cerebral cortex. Nat Commun. 13 (1), 6571 (2022).
- Tabata, H., Nakajima, K. Multipolar Migration: The third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 23 (31), 9996-10001 (2003).
- Tabata, H., Kanatani, S., Nakajima, K. Differences of migratory behavior between direct progeny of apical progenitors and basal progenitors in the developing cerebral cortex. Cereb Cortex. 19 (9), 2092-2105 (2009).
- Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 145 (1), 61-83 (1972).
- Sekine, K., Honda, T., Kawauchi, T., Kubo, K., Nakajima, K. The outermost region of the developing cortical plate is crucial for both the switch of the radial migration mode and the Dab1-dependent "inside-out" lamination in the neocortex. J Neurosci. 31 (25), 9426-9439 (2011).
- Shin, M., et al. Both excitatory and inhibitory neurons transiently form clusters at the outermost region of the developing mammalian cerebral neocortex. J Comp Neurol. 527 (10), 1577-1597 (2019).
- Sekine, K., et al. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1. Neuron. 76 (2), 353-369 (2012).
- Morimoto, K., Tabata, H., Takahashi, R., Nakajima, K. Interactions between neural cells and blood vessels in central nervous system development. BioEssays. 230091, (2023).
- Tabata, H., Nagata, K. Decoding the molecular mechanisms of neuronal migration using in utero electroporation. Med Mol Morphol. 49 (2), 63-75 (2016).
- Ishii, K., Kubo, K., Nakajima, K. Reelin and neuropsychiatric disorders. Front Cell Neurosci. 10, 229 (2016).
- Bosworth, A. P., Allen, N. J. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol. 47, 38-43 (2017).
- Tabata, H. Crosstalk between blood vessels and glia during the central nervous system development. Life. 12 (11), 1761 (2022).
- Wiegreffe, C., Feldmann, S., Gaessler, S., Britsch, S. Time-lapse confocal imaging of migrating neurons in organotypic slice culture of embryonic mouse brain using in utero electroporation. J Vis Exp. (125), e55886 (2017).
- Saito, T., Nakatsuji, N. Efficient Gene Transfer into the Embryonic Mouse Brain Using in Vivo Electroporation. Dev Biol. 240 (1), 237-246 (2001).
- Tabata, H., Nakajima, K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ. 50 (6), 507-511 (2008).
- Tabata, H., Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neurosciences. 103 (4), 865-872 (2001).
- Fukuchi-Shimogori, T. Neocortex patterning by the secreted signaling molecule FGF8. Science. 294 (5544), 1071-1074 (2001).
- Matsumoto, K., et al. Study of normal and pathological blood vessel morphogenesis in Flt1-tdsRed BAC Tg mice. Genesis. 50 (7), 561-571 (2012).
- Kawai, S., Takagi, Y., Kaneko, S., Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim. 60 (5), 481-487 (2011).
- Meijering, E., Dzyubachyk, O., Smal, I. Methods for cell and particle tracking. Methods Enzymol. 504, 183-200 (2012).
- Ershov, D., et al. TrackMate 7: Integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat Methods. 19 (7), 829-832 (2022).
- Tinevez, J. -. Y., et al. TrackMate: An open and extensible platform for single-particle tracking. Methods. 115, 80-90 (2017).
- Gloster, A., et al. The T alpha 1 alpha-tubulin promoter specifies gene expression as a function of neuronal growth and regeneration in transgenic mice. J Neurosci. 14 (12), 7319-7330 (1994).
- Zhuo, L., et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 31 (2), 85-94 (2001).
- Yusa, K., Zhou, L., Li, M. A., Bradley, A., Craig, N. L. A hyperactive piggyBac transposase for mammalian applications. P Natl Acad Sci USA. 108 (4), 1531-1536 (2011).
- Chen, F., LoTurco, J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J Neurosci Meth. 207 (2), 172-180 (2012).
- Wang, X., Qiu, R., Tsark, W., Lu, Q. Rapid promoter analysis in developing mouse brain and genetic labeling of young neurons by doublecortin-DsRed-express. J Neurosci Res. 85 (16), 3567-3573 (2007).
- Sauer, B. DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Res. 32 (20), 6086-6095 (2004).
- Hermann, M., et al. Binary recombinase systems for high-resolution conditional mutagenesis. Nucleic Acids Res. 42 (6), 3894-3907 (2014).
- Kanatani, S., et al. The COUP-TFII/Neuropilin-2 is a molecular switch steering diencephalon-derived GABAergic neurons in the developing mouse brain. P Natl Acad Sci USA. 112 (36), E4985-E4994 (2015).
- Yozu, M., Tabata, H., Nakajima, K. The caudal migratory stream: A novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 25 (31), 7268-7277 (2005).
- Kanatani, S., Yozu, M., Tabata, H., Nakajima, K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 28 (50), 13582-13591 (2008).
- Kitazawa, A., et al. Hippocampal pyramidal neurons switch from a multipolar migration mode to a novel "climbing" migration mode during development. J Neurosci. 34 (4), 1115-1126 (2014).

