- 00:00Vue d'ensemble
- 01:28Principles of Soil Nutrient Analysis
- 04:12Extraction of Nutrients
- 06:21Analyzing Samples for Nitrate
- 07:52Analyzing Samples for Phosphate
- 09:16Analyzing Samples for Potassium
- 11:13Applications
- 13:08Summary
土壤养分分析: 氮、 磷和钾
English
Diviser
Vue d'ensemble
资料来源: 玛格丽特工人和金伯利弗莱-Depaul 大学实验室
在这个实验中,三种土壤营养化学提取,结合基于颜色的试剂,然后分析了利用颜色测定土壤样品中的养分含量。
氮、 磷和钾是土壤肥料的主要组成部分。这些方法隔离每个从土壤养分到解决方案中,可以使用浊度和颜色来确定营养与土壤样品中的浓度进行了分析。了解目前浓度通知的一种营养缺乏或盈余用于支持植物生产的土壤中的环境科学家和也提供基本的生态系统的生物地球化学循环的一般洞察。
Principles
当化学分离土壤,营养素可以会检测到使用这种技术。氮和磷,通常发现的硝酸盐和磷酸盐,形式,提取出化学萃取剂,将绑定养分的兴趣了。一旦从土壤中提取,每种营养成分可以结合一种已知的试剂,导致营养液来更改为中的线性关系,用深色指示养分浓度增加养分特定颜色。若要分析每种营养成分的浓度,一种化学试剂将用于颜色随每个样本的颜色强度指示养分浓度增加。
在高和中程硝酸盐测试中,金属镉用来减少硝酸盐 (没有3–) 对亚硝酸盐 (没有2–)。镉购买的 Nitraver 5 (高和中等范围) 和 Nitraver 6 (小范围) 所载粉枕头。
没有3– + Cd + 2 H+ →无2– + Cd2 + + H2O
亚硝酸盐离子然后形成中间重氮盐与对氨基苯磺酸酸 (在酸性介质中载 NitraVer 5 粉) 反应。当加龙胆酸 (也载于 NitraVer 5),形成一种琥珀色的解决方案。 这种化合物的颜色强度水样的硝酸浓度成正比,和硝酸颜色比较器框采用连续硝酸盐琥珀色磁盘是可以量化。
磷、 钠钼和购买的 PhosVer 3 试剂粉末硫酸钾与可溶性活性磷酸盐形成一个复杂的磷钼酸盐的反应。
H2宝4– + 12 Na MoO24 + → PMo12O403-
抗坏血酸 (也载于 PhosVer 3 粉) 然后减少复杂,形成一种钼蓝颜色。蓝色被量化与连续磷酸蓝色磁盘使用磷酸颜色比较器框。
彩盒比较器使用本方法。此工具运行基于已知的颜色强度为 0-50 毫克之间每个浓度/L.在框中的颜色磁盘打开直到两个查看窗口中的颜色 (空白和样品) 的比赛。一旦颜色相匹配,相应的养分浓度 (mg/L) 将显示在一个单独的低窗口颜色比较器框上。这些箱足够健壮,用于在任何水平大学入门课程的学生,很容易可以运输作为检测试剂盒可以在取样位置使用农田土壤的一部分。这些方法允许基本营养课堂在实验室中测试而无需昂贵件可能不可用的设备。确保测试精度、 硝酸盐和磷酸盐标准溶液可以代替程序之前前往现场或开始在实验室中的土壤样品分析中的样品。
在钾测试中,钾离子结合形成白色沉淀四苯硼酸钾购买钾 3 试剂粉所载四苯硼钠。沉淀仍在悬浮在样品,造成浊度增加。
没有3– + Cd + 2 H+逮捕 (C6H5)4 + K+ → KB (C6H5)4 ++ Na
钾测量尺用来量化的转换为钾离子浓度的浊度。量油尺放置在样品,直至点不再是通过白色沉淀物可见的另一端有一个黑点。量油尺增量标记以指示规模再转换成钾浓度与转换图表的可见性。这种方法是使用最小的设备,可以被运输到室外采样站点和足够强劲,用于在任何水平大学入门课程的学生便宜的程序。
Procédure
Résultats
Each nutrient analysis will result in a concentration reported in mg/L.
Nitrate and Phosphate concentrations will be determined with the color comparator boxes and display the result in the window.

Figure 1. Example color disks for nitrate (left) and phosphate (right) color comparator boxes. Color intensities are on the outer edge of the disks and nutrient concentration (mg/L) are on the inner edge of the disks.
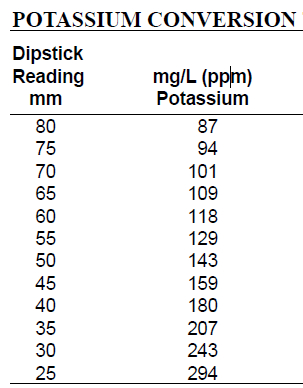
Table 1. Potassium Conversion Table used to convert dipstick potassium reading into mg/L. Locate the dipstick reading on the left column and record the corresponding mg/L concentration on the right column.
| Nitrogen | Phosphorus | Potassium | |
| Nutrient level range in ppm | |||
| Low | 0-15 | 0-25 | 0-60 |
| Medium | 15-30 | 25-50 | 60-100 |
| High | 30+ | 50+ | 100+ |
Table 2. Table of nutrient ranges arranged by categories.
Applications and Summary
Determining the nutrient concentrations for nitrate, phosphates, and potassium can reveal how a soil is functioning in regards to its intended use and how nutrients are cycling through a soil. A nutrient test provides a report of average nutrient concentration (mg/L) for all nutrients tested. In an agricultural setting, knowing the concentration of nutrients can help food producers know when to add fertilizer, how much to add, and which nutrients need supplemented and in what amount. Consistently high nitrogen soils, for instance, would be good for growing nitrogen-demanding crops such as soy and corn. High nitrogen levels are also particularly useful for non-flowering plants because nitrogen is required for any green part of plants. High nitrogen levels can suppress flowering however, if they remain higher than phosphorus levels. Phosphorus controls flowering in plants and is important to any plant production involving flowering or fruiting plants and phosphorus is often added to soils or directly to plants before and during flowering and fruiting life-cycle stages to increase agricultural yields in larger crop size and increased amounts of fruit production per plant. Potassium is involved in catalyzing many chemical reactions required to support plant life including drought tolerance and moisture regulation. Low potassium soils will likely need to be irrigated if soil amendment is not possible. Nutrient concentration can also inform of nutrient deficiencies or surpluses that can be detrimental to plant growth. If a nutrient is too high, amendments can be performed to reduce a surplus, such as adding mulch or tilling the soil. If nutrients are too low to support plant production, fertilization can be used to add nutrients in an amount needed for a specific crop. Low nutrient soil may also have more applicable uses to land managers for recreational or developed (paved surfaces or building construction) spaces.
Transcription
Soil nutrient analyses can be carried out to extract three major soil macronutrients, nitrogen, phosphorus, and potassium, and combine them with color-based reagents to determine their concentration.
Nitrogen, phosphorus, and potassium are major components of soil fertilizer. Knowing their concentration in soils can inform environmental scientists of nutrient deficiency or surplus in soils used to support plant production, and provide a general insight into the basic biogeochemical cycles of an ecosystem.
Soil nutrient analysis can be carried out using chemicals to bind the macronutrient of interest. For nitrogen or phosphorus, reagents are added which react to the presence of the specific macronutrient and produce colored products. Potassium concentration is determined by forming precipitates in an amount proportional to potassium concentration.
These methods are simple, inexpensive, require minimal equipment, and can be carried out in a field setting if desired. This video will illustrate the techniques used to extract and quantify these common soil macronutrients.
To begin analysis, macronutrients are first extracted from collected soil samples. Nitrogen is extracted using calcium sulfate; phosphorus and potassium are extracted using Mehlich 2 solution, a solution of acetic acid, ammonium chloride, hydrochloric acid, hydrofluoric acid, and demineralized water.Boundmacronutrients present in suspension can then be separated from the remaining solid soil components by vacuum filtration.
Once macronutrients have been extracted, their concentration can be determined. For nitrogen, cadmium metal is used to reduce nitrates to nitrites. This cadmium is present in pre-packaged pillows that are added to the soil filtrate. The nitrite ions react with sulfanilic acid to form diazonium salt. This couples with gentisic acid and an amber solution is formed.
For phosphorus, sodium molybdate reacts with the soluble reactive phosphate to form a phospho-molybdate complex. This is then reduced by ascorbic acid to form a molybdenum blue color.
The color intensity of both solutions is proportional to the nutrient concentration. Color comparator boxes are used for analysis of nitrate and phosphate. Samples are compared to a blank, and the color disk is turned until both viewing windows match. The corresponding nutrient concentration in mg/L will be displayed in a separate window. The color intensity of both solutions is proportional to the nutrient concentration.
To quantify potassium, the ions from the soil filtrate combine with sodium tetraphenylborate to form potassium tetraphenylborate, a white precipitate. The precipitate remains in suspension, causing an increase in turbidity.
A potassium dipstick is used to quantify turbidity caused by the precipitate. The dipstick is placed in the sample and lowered until the black dot at the end is no longer visible. The stick is incrementally marked, and readings on this scale can be converted to potassium concentration using a conversion chart.
Now that we are familiar with the principles behind extraction and quantification of soil macronutrients, let’s take a look at how the procedures are carried out in the laboratory.
Once the soil samples have been collected, correctly transported, and stored, they can be brought into the laboratory for analysis, beginning with the nitrogen extraction. First, turn on the balance, set a weigh boat on top, and tare.
Using a spatula, weigh out 10 g of dried, sieved soil sample and transfer to a labeled 100-mL beaker. Next, weigh 0.1 g of calcium sulfate and transfer it to the beaker.
Measure out 20 mL of deionized water with a graduated cylinder and transfer to the beaker. Thoroughly mix the contents of the beaker with a stirring rod. Repeat these additions for each test soil sample. Secure samples on a tabletop shaker and agitate for 1 min.
To begin extraction of phosphorus and potassium from the soils, use a spatula to weigh out 2 g of dried, sieved soil sample, and transfer to a labeled 100-mL beaker. With a graduated cylinder, measure 20 mL of Mehlich 2 soil extractant and transfer to the beaker. Thoroughly mix the contents of the beaker with a stir rod. Secure samples on a tabletop shaker and agitate for 5 min. After extraction, all three nutrient sample sets should be vacuum filtered using a vacuum flask and Büchner funnel.
First, turn on the vacuum jet and slowly pour the soil extract solution into the funnel. Extract should drain from the funnel, into the flask. Pour the filtrate into a clean, labeled 50-mL beaker. Remove the funnel, discard filter paper, and rinse funnel and flask with deionized water. Use an air jet to dry the funnel and flask.
Now the nutrient samples have been filtered, content analysis can begin. For each nutrient test, begin by labeling a color viewing tube with an “S”, for sample. Label a second with a “B” for blank.
Thoroughly rinse both color viewing tubes with deionized water, then shake to remove the remaining rinse water. Add the sample extract to a depth of ¼ inch in the color viewing tube marked “S”. Cap the tube with a rubber stopper and shake for 3 s, then discard the solution.
Next, add the sample extract to both tubes until the meniscus is even with the 5-mL mark on the tubes, at the bottom of the frosted area. Add the contents of one nitrogen reagent pillow to the tube marked “S”. Cap and shake the tube vigorously for 1 min. Immediately place both tubes into the comparator, with tube “B” in the outside hole, and tube “S” on the inside. Leave for 5 min.
Hold the comparator up to a light source and rotate the disc until the color in the window for tube “B” matches that in the window for tube “S”. Record the concentration value displayed in the lower window of the color comparator box.
Samples can also be analyzed for phosphate content using the color comparator. Using a dropper, add 2.5 mL of the filtered phosphorus sample extract to a 25 mL graduated cylinder. Add deionized water to the 25 mL mark, cap with a stopper, and invert to mix. Add the diluted sample extract to about ¼ inch deep in the color viewing tube marked “S” to rinse the tube. Cap with a rubber stopper, and shake for a few seconds before discarding the solution.
Into both tubes, add the sample extract until the meniscus is even with the 5 mL mark. Add the contents of one phosphorus reagent pillow to the “S” tube, cap, and shake vigorously for 1 min. Immediately place the color tubes into the color comparator, with the blank tube in the outside hole, and the sample tube in the inside hole. Leave for 3 min. Hold the comparator up to a light source, and rotate the disc until the window for tube “B” matches the color in the window for tube “S”. Record the value displayed in the window.
Finally, samples can be analyzed for potassium content. Using a dropper, add 3 mL of potassium sample extract to a 25 mL cylinder. Add deionized water to the 21 mL mark on the cylinder, cap firmly with a rubber stopper, and invert. Next, add one potassium 2 reagent pillow to the cylinder. Add 3 mL of an alkaline EDTA solution to the cylinder, cap with a rubber stopper, and invert several times to mix. Let the solution stand for 3 min. Add the contents of one potassium reagent pillow, cap the cylinder and shake vigorously for 10 s. Allow the solution to stand for 3 min as a white turbidity develops.
Looking straight down into the cylinder, slowly insert the potassium dipstick vertically into the solution until the black dot is no longer visible from above. Hold the dipstick in position and rotate the cylinder to view the scale. Record the number on the dipstick scale where the surface of the sample meets the dipstick. Refer to the potassium conversion table to determine the concentration of samples in mg/L. Locate the dipstick reading in the left hand column, and record the corresponding mg/L concentration reported in the right hand column.
Once concentrations are obtained, a table of nutrient ranges can be used to assess sample quality and determine whether sampled soil needs nutrient amendment, and if so, how much. Nutrient amendment can be carried out by application of specific fertilizers.
The ability to analyze the soil nutrient composition of soils has a wide variety of applications, with potential implications for human populations or agricultural ecosystems.
Different crop plants will have different potential nutrient requirements for optimal growing. For example, high nitrogen levels are needed for growing nitrogen-demanding crops, such as soy and corn. High levels of phosphorus can stimulate and enhance flower or fruit production. The ability to measure soil nutrient composition in an intended crop growing area can therefore allow farmers or land managers to supplement the soil with necessary nutrients to grow their intended crop successfully.
The composition of soil can also have implications for its ability to retain water, which can in turn influence its ability to support different flora or fauna. For example, low potassium soils have poor drought tolerance, and may require nutrient amendment, by fertilization of the soil with appropriate amounts of the missing nutrient. Alternatively, irrigation may be necessary to grow any plants that do not display high drought tolerance.
Soil composition and nutrient quality can also help inform land managers to designate appropriate land-use. In areas where the soil has poor nutrient quality, that would require heavy modification or supplementation to grow crop plants, setting aside land for development of buildings or structures may be more appropriate. Alternatively, areas with ideal composition for intended crop growing can be earmarked and set aside, protected from development.
You’ve just watched JoVE’s introduction to Soil Nutrient Analysis. You should now understand the importance of soil macronutrients, how to extract them from soils, and how to determine their concentrations. Thanks for watching!




