X 線回折用結晶を成長
English
Diviser
Vue d'ensemble
ソース: 博士ジミー フランコ – メリマック大学講座
X 線結晶構造解析、分子または複雑な三次元形状の決定は、結晶性固体中の原子の立体配置を決定するために一般的に使用される方法です。化合物の構造と機能は密接に関連するので特に重要です、化合物の立体構造を決定します。化合物の構造についての情報は、その動作や反応を説明するためによく使用されます。これは、化合物の立体構造を解決するための最も有用な技術の 1 つまたは複雑で、場合によっては構造の決定の唯一の現実的な方法があります。X 線質の結晶成長は、x 線結晶構造解析の重要なコンポーネントです。サイズと結晶の品質は、x 線結晶構造解析で検討されている化合物の組成に依存されます。通常より重い原子を含む化合物は大きい回折パターンを作り出す、従ってより小さい結晶が必要があります。一般的には、明確に定義された面を持つ単結晶が最適と通常有機化合物の結晶は重原子が含まれているものよりも大きくなければなりません。実行可能な結晶は、なし、x 線結晶構造解析は不可能です。いくつかの分子が他の人よりも本質的により結晶、従って x 線品質の結晶を得ることの難しさは、化合物によって異なります。X 線結晶の成長は、高品質の結晶を製造に重点を置いたが、化合物を浄化するために使用される再結晶過程に似ています。多くの場合、高品質結晶は結晶化のプロセス遅く、日または数か月にわたって発生することがありますを許可することで取得できます。
Principles
Procédure
Résultats
The technique of liquid-liquid diffusion was used to create X-ray quality crystals of tetraphenylporphyrin. Using dichloromethane as the solvent and methanol as the anti-solvent, the liquids were allowed to slowly diffuse over the course of a week without being disturbed. Large, well-defined, dark purple-reddish crystals formed at the interface of the two solvents (Figure 3). The growth of the crystals can be visually observed. The crystals grew with very well defined faces, which can be seen with a microscope.
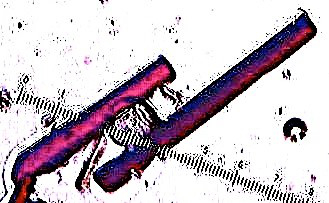
Figure 3. X-ray diffraction quality crystals of TPP. Crystals that are clumped together or that are growing out of one another should be avoided. Large single crystals with well-defined faces typically yield better results.
Applications and Summary
X-ray quality crystals can be grown by liquid-liquid diffusion. The slow diffusion of the binary solvent system allows for the creation of crystals suitable for X-ray diffraction.This method allows the crystal lattice to form slowly, often leading to larger and more well defined crystals. The use of NMR tubes facilitates the slow diffusion of the solvents, allowing for optimal crystal growth. This process can take anywhere from a few days to several months. Often during the crystallization process solvent molecules are incorporated into the crystal lattice. Thus it is important to avoid allowing the crystals to "dry out". Thus, one of the advantages of liquid-liquid diffusion is that the crystals typically grow at the interface of the two solvents, which circumvents this phenomenon.
Liquid-liquid diffusion is one of the most useful techniques for producing X-ray quality crystals, which is the most essential component of X-ray crystallography. Obtaining X-ray quality crystals is typically the limiting factor on conducting X-ray crystallography experiments. X-ray crystallography essentially creates a three-dimensional picture of a molecule's structure, making it the least ambiguous method for determining the complete configuration of a compound. Since structure and function of molecules are intimately related, the ability to decipher a compound's three-dimensional structure is extremely useful for a variety of chemical and pharmaceutical applications. Researchers and pharmaceutical companies use X-ray crystallography to determine the structure of proteins to examine how small molecules interact with enzymes for the purpose of drug discovery and design.3-5 X-ray crystallography is also one of the most useful methods for evaluating metal complexes. This technique divulges valuable insight on how metals interact with each other and its ligands. The first ever identified quintuple bond between two chromium atoms was identified using X-ray crystallography.6 This technique can also be used to explain the luminescent properties of metal complexes.7 Crystallography has also been widely used in host-guest chemistry, as this method has been instrumental in revealing valuable information about non-covalent interactions between molecules.8
References
- Gilman, J. J., The art and science of growing crystals. Wiley: (1963).
- Orvig, C., A simple method to perform a liquid diffusion crystallization. Journal of Chemical Education 62 (1), 84 (1985).
- Brown, C. S.; Lee, M. S.; Leung, D. W.; Wang, T.; Xu, W.; Luthra, P. et. al. In silico derived small molecules bind the filovirus VP35 protein and inhibit its polymerase co-factor activity. Journal of molecular biology, 426 (10), 2045-2058 (2014)
- Batt, S. M.; Jabeen, T.; Bhowruth, V.; Quill, L.; Lund, P. A.; Eggeling et. al. Structural basis of inhibition of Mycobacterium tuberculosis DprE1 by benzothiazinone inhibitors. Proceedings of the National Academy of Sciences of the United States of America,109 (28), 11354-9 (2012)
- Mortensen, D. S.; Perrin-Ninkovic, S. M.; Shevlin, G.; Elsner, J.; Zhao, J.; Whitefield et al. Optimization of a Series of Triazole Containing Mammalian Target of Rapamycin (mTOR) Kinase Inhibitors and the Discovery of CC-115. Journal of Medicinal Chemistry 58 (14), 5599-5608 (2015)
- Nguyen, T.; Sutton, A. D.; Brynda, M.; Fettinger, J. C.; Long, G. J.; Power, P. P., Synthesis of a Stable Compound with Fivefold Bonding Between Two Chromium(I) Centers. Science310 (5749), 844-847 (2005).
- Chen, K.; Nenzel, M. M.; Brown, T. M.; Catalano, V. J., Luminescent Mechanochromism in a Gold(I)-Copper(I) N-Heterocyclic Carbene Complex. Inorganic Chemistry 54 (14), 6900-6909.(2015).
- Franco, J. U.; Hammons, J. C.; Rios, D.; Olmstead, M. M., New Tetraazaannulene Hosts for Fullerenes. Inorganic Chemistry49 (11), 5120-5125 (2010).
Transcription
A single crystal is required for the determination of its structure. The quality of the crystal heavily influences the quality and accuracy of the structural determination.
A single crystal is a solid in which the molecule arrangement repeats in all three dimensions. The spatial arrangement of the atoms within the crystalline solid can be determined using X-ray crystallography. In this technique, a pure crystalline sample is enveloped by a beam of X-rays. The crystal diffracts the X-rays in a distinctive pattern related to the crystals structure and molecular composition. If a crystal is formed too quickly, the molecules may be disordered, impurities may be incorporated into the crystal, or two or more fused crystals may form instead of a single crystal. Therefore, specialized methods with emphasis on slow growth are needed to produce crystals of sufficient quality for X-ray crystallography.
This video will illustrate the desired characteristics of X-ray quality crystals, demonstrate a procedure for growing them, and introduce a few applications of this technique in chemistry.
Electrons scatter X-rays by emitting a spherical X-ray wave when hit. If the atoms are in an orderly arrangement, constructive interference between the waves produces a characteristic diffraction pattern on an X-ray detector. The crystal is rotated within the beam to collect diffraction patterns from multiple angles. With sufficient diffraction patterns, the molecular structure can be derived.
X-ray-quality crystals generally form symmetric shapes and have smooth, light-reflecting faces. When viewed under a polarizing microscope, they will be transparent, but most should become dark when rotated 90°. This indicates highly-ordered structure. To grow these crystals, liquid-liquid diffusion is often used. This employs two miscible solvents: a low-density solvent, or precipitant, in which the compound to be recrystallized is insoluble; and a high-density solvent in which the compound is soluble. Typically, the volumetric ratio of precipitant to solvent is 2:1.
The low-density precipitant is layered onto a concentrated solution of the compound in the high-density solvent. Over time, the compound becomes less soluble as the precipitant mixes with the solution. A smaller solvent interface results in a slower rate of diffusion, thus yielding larger, purer crystals. Now that you understand the principles of growing X-ray quality crystals, let’s go through a procedure for growing them by liquid-liquid diffusion.
To begin, obtain the necessary equipment found in the text protocol. Acquire a solvent for the compound and a less dense precipitant.
To prepare a pipette filter, place a small piece of Kimwipe into the top of a glass pipette and gently press the paper down to the bottom of the pipette body using a rod or the stem of another pipette, being careful not to puncture the paper. Prepare two pipette filters. Place one into the NMR tube. If necessary, secure the assembly with a laboratory clamp and ring stand. Dissolve about 10 mg of the compound to be recrystallized in 0.75 mL of solvent.
Now, carefully add the sample solution into the pipette filter. Affix a bulb to the top and slowly squeeze to pass the solution into the NMR tube to remove solid impurities. Do not allow the bulb to re-expand while it is attached, as the suction will dislodge the filter paper.
Next, remove the used pipette filter and place the second filter into the NMR tube. Pipette approximately 1.5 mL of precipitant into the tube. Allow the solvent to pass through the filter by gravity. From now on, take care not to disturb the filter during any manipulations. Once all of the precipitant has filtered into the tube, remove the filter and cap the tube. Place it in a cabinet or other easily checked location where it will not be agitated.
After at least one day, inspect the tubes for crystal growth. If no crystals are present or the crystals are very small, leave the sample tube undisturbed. If crystals are visible, check their size and shape without disturbing the solvent layers.
If the crystals are large, well-defined, and are not clustered together, inspect the crystals under a microscope to verify their potential to be X-ray quality. Do not remove the crystals from the tube until the diffractometer is ready to begin the scan. If solvent molecules are incorporated into the crystal structure, allowing the crystal to dry will degrade the crystal. Using X-ray crystallography, the molecular structure of these dark reddish-purple crystals was verified to be tetraphenylporphyrin.
X-ray crystallography is an essential analytical tool in chemistry and biochemistry.
Recrystallization methods include heating and cooling, liquid-liquid diffusion, vapor diffusion, and slow evaporation. In slow evaporation of a single solvent system, the compound is dissolved in a small amount of solvent and placed in a container with a small hole in the cap. As the solvent evaporates, the concentration increases until the compound begins crystallizing.
The functionality of proteins is often related to their structure. However, proteins can be very difficult to crystallize. Specialized techniques must be developed to grow X-ray-quality crystals of proteins. Here, a drop of protein solution is mixed with a drop of precipitant and this mixture is sealed in a chamber with pure precipitant. As the solvent vapor diffuses out of the drop, the solubility of the protein in the drop decreases, and the protein slowly crystallizes. Another technique mixes the protein solution and precipitant under mineral oil. Using these techniques, a variety of proteins can be crystallized for analysis.
In powder diffraction, each possible spatial orientation is represented in the sample simultaneously. Powder diffraction is not as informative about structure as single crystal X-ray diffraction because of the loss of three-dimensional structure data. Instead, powder diffraction excels in analyzing mixtures of crystalline solids and assessing the crystallinity of amorphous structures.
You’ve just watched JoVE’s introduction to growing crystals for X-ray crystallography. You should now be familiar with the properties of X-ray quality crystals, a procedure for growing them, and a few applications of this technique in chemistry.
Thanks for watching!
