- 00:00Vue d'ensemble
- 01:18Lewis Acid-Base Interactions in Ph3P-BH3
- 03:15Schlenk Line Set Up
- 03:54Synthesis of Borane Triphenylphosphine Complex
- 05:39Work Up, Isolation, and 31P-NMR
- 06:21Résultats
- 07:08Applications
- 08:37Summary
루이스 산-베이스 상호 작용 Ph3P-BH3
English
Diviser
Vue d'ensemble
출처: 타마라 M. 파워스, 텍사스 A&M 대학교 화학학과
화학의 목표 중 하나는 추세를 설명하고 반응성에 기여하는 반응제의 특성에 대한 통찰력을 제공하는 모델을 사용하는 것입니다. 물질은 고대 그리스 시절부터 산과 기지로 분류되었지만 산과 기지의 정의는 수년에 걸쳐 수정되고 확장되었습니다. 1
고대 그리스인들은 맛에 의한 물질을 특성화하고, 레몬 주스와 식초와 같은 신맛으로 산을 정의했습니다. 용어 “산”은 라틴어 용어에서 파생되어 “신 맛”을 위해 파생됩니다. 염기는 산을 중화하거나 중화시키는 능력을 특징으로했다. 특징의 첫 번째 기지는 비누를 만들기 위해 지방과 혼합 된 화재에서 재의 그였다. 사실,용어 “알칼리성”은 아랍어 단어에서 파생되어 “로스팅”을 위해 파생됩니다. 실제로, 산과 기지를 결합하여 소금과 물을 줄 수 있다는 것이 고대부터 알려져 왔습니다.
산의 첫 번째 널리 사용되는 설명은 스웨덴 화학자, Svante Arrhenius의 것입니다, 누가 1894 년에 하이드로늄 이온을 제공하기 위해 물에 해리 물질로 산을 정의하고, 수산화 이온을 제공하기 위해 물에 해리 물질로 염기. 따라서 이러한 정의는 수성산으로 제한되며 산이 양성자에게 기여하는 것이 필요하다. 2 예를 들어, 물에서 HCl은 하이드로늄 이온(H3O)+ 및 염화물 이온을 제공하기 위해 해리화되기 때문에 산이다. 보론 트리클로라이드는 물에서와 같이 산으로 간주되지 않을 것이며, 물에서와 같이 B (OH)3 및 3 HCl을 제공하기 위해 가수 분해합니다. 제품 HCl 하지만 아레니우스 산.
1923년 요하네스 니콜라우스 브뢰스테드와 마틴 로리(Martin Lowry)는 수소 이온 또는 양성자를 기증하고 받아들이는 능력에 대해 산과 기지를 독립적으로 정의했습니다. 따라서 산염-염기 공수 쌍의 개념이 나왔고, 물 이외의 용매에서 산및 염기의 정의의 확장이 이루어졌다. 예를 들어, 암모늄은 양성자를 기증하고 암모니아를 생성할 수 있기 때문에 산입니다. 암모니아는 암모늄을 제공하기 위해 양성자를 받아 들일 수 있습니다. 따라서 암모니아는 암모늄의 컨쥬게이트 기저입니다. 이 산염 염기 반응은 물, 암모니아 또는 그밖 용매에서 생길 수 있습니다.
이 비디오는 1923년에 산과 기지를 정의한 미국 화학자 길버트 N. 루이스의 산염 기본 정의를 다룹니다. 사실, 이것은 일반 화학에서 루이스 도트 구조에서 같은 루이스입니다. 그의 접근 방식은 양성자를 기증하고 받아들이는 산과 기지의 능력이 아니라 전자 쌍을 각각 받아들이고 기증하는 능력에 초점을 맞추고 있습니다. 이것은 H+가 프로토 네이션 동안 Brønsted 기지에서 전자 쌍을 받아 들이기 때문에 Brønsted-Lowry 정의를 포함합니다. 그러나, 그것은 크게 산의 정의를 확장, 지금 금속 이온 및 주요 그룹 화합물을 포괄. 여기에서, 우리는 루이스 산염 제합 부관 Ph3P-BH3의 3PNMR을 무료 트리페닐포스핀과 비교합니다.
Principles
Procédure
Résultats
Borane triphenylphosphine complex:
31P NMR (chloroform-d, 500 MHz, δ, ppm): 20.7 (broad doublet)
Triphenylphosphine:
31P NMR (chloroform-d, 500 MHz, δ, ppm): -5.43
The 31P NMR signal of the borane triphenylphosphine complex is downfield relative to free triphenylphosphine. This is consistent with removal of electron density from the phosphorous center, which is deshielded upon adduct formation.
Applications and Summary
The borane triphenylphosphine complex is an example of a Lewis-adduct, whereby a Lewis base donates electrons to a Lewis acid. Though BH3 and PPh3 would not necessarily be considered an acid and base, respectively, using other acid-base theories, Lewis acid-base theory predicts correctly that the molecules form a stable adduct.
Small Molecule Activation:
While transition metal ions have historically been regarded as Lewis acids, the notion that they can serve as Lewis bases is being advanced. For example, Jonas Peters and co-workers at Caltech have shown that metal-borane complexes, which can donate electrons to the Lewis acid borane (a Z-type ligand), can give rise to novel reactivity. A nickel borane species was shown to reversibly add H2, heterolytically cleaving the H-H bond.4 The H2-added species is a catalyst for hydrogenations of olefins. The group also reported that iron-borane complexes can catalytically reduce nitrogen to ammonia.5 This was the first example of an iron-based homogeneous catalyst for this challenging yet critical reaction.
Frustrated Lewis Pairs:
Another current area of research is that of "Frustrated Lewis Pairs," or FLPs. These are Lewis acid-base "adducts" that due to steric reasons, cannot form a dative bond.6 Douglas Stephan and co-workers from the University of Toronto pondered what reactivity such adducts would have, particularly with the idea of using them for small molecule activation and catalysis. Thinking about transition metal complexes, which can both accept and donate electron density to and from substrates, they hypothesized donor/acceptor properties of what they termed "Frustrated Lewis Pairs" might have with regards to reactivity.
In 2006, Stephan and co-workers reported in Science that the zwitterionic (C6H2Me3)2PH(C6F4)BH(C6F5)2 reversibly loses H2 to give (C6H2Me3)2P(C6F4)B(C6F5)2.7 This was the first example of reversible H2 activation with main group elements, and other examples followed (Figure 3). This study paved the way for the development of FLP research. Since then, FLPs have been developed that are competent hydrogenation catalysts, and can activate a variety of small molecules including CO2. This is an active and exciting new area of research.
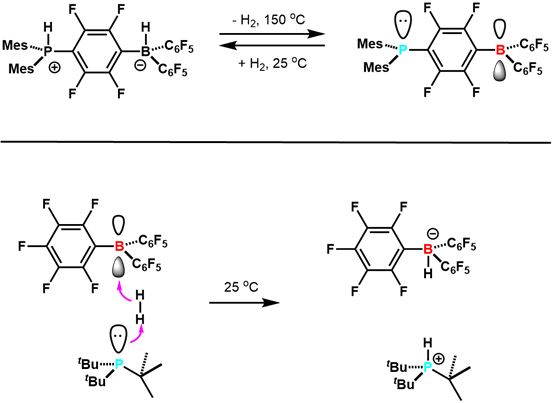
Figure 3. Early examples of reactivity of FLPs with H2. Adapted from reference 5.
References
- Lesney, Today's Chemist at Work, 2003, 47-48.
- Miessler, P. J. Fischer and D. A. Tarr, Inorganic Chemistry, Pearson, 2014.
- McNulty, J.; Zhou, Y. Tetrahedron Letters, 2004, 45, 407-409.
- Harman and J. C. Peters, J. Am. Chem. Soc., 2012, 134, 5080-5082.
- Anderson, J. Rittle and J. C. Peters, Nature, 2013, 501, 84-87.
- Stephan, J. Am. Chem. Soc., 2015, 137, 10018-10032.
- Welch, R. R. S. Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124-1126.
Transcription
In chemistry, acid-base models are used to explain trends in reactivity and characteristics of reactants, which is important when designing a synthesis.
In 1894, Svante Arrhenius pioneered the concept of acids and bases, describing them specifically as substances that dissociate in water, to yield hydronium or hydroxide ions, respectively.
In 1923, Johannes Brønsted and Thomas Lowry defined acids and bases by their ability to donate and accept hydrogen ions in different solvents, creating the concept of acid-base conjugate pairs.
In the same year Gilbert Lewis proposed an alternative, defining acids and bases by their abilities to donate and accept electron pairs, instead of protons. This model expanded the application of acids and bases, taking into account metal ions and main-group compounds.
This video will illustrate the Lewis acid-base concept on the basis of a triphenylphosphine borane complex, its synthesis, and analysis.
When using the Lewis Acid-and Base model, the molecular structure needs to be considered to identify whether the molecule will donate or accept an electron pair.
Therefore, start with the structure analysis of triphenylphosphine and borane using the VSEPR theory, and then determine the Lewis acid and base.
Triphenylphosphine has three covalent bonds between the phosphorous atom and a carbon in each of the three phenyl rings. Two free electrons are left as a free electron pair to fill the octet.
Furthermore, triphenylphosphine is sp3 hybridized at the phosphorous center and has a tetrahedral electronic geometry. The lone-pair of electrons residing in an sp3 orbital can be donated to another molecule, classifying triphenylphosphine as a Lewis base.
On the other hand, borane has three covalent bonds between the boron and the three hydrogen atoms. Since the borane center has only six valence electrons it does not fulfill the octet rule and is therefore electron-deficient.
The geometry is trigonal planar and the bonds are sp2 hybridized. The lone p orbital is empty, and ready to accept electrons, which classifies borane as a Lewis acid.
If triphenylphosphine donates its two electrons to the empty p orbital in borane, it leads to a change of the hybridization from sp2 to sp3 and one can propose that a stable Lewis acid-base adduct will form.
This type of bond between a Lewis-acid and base is often called a coordinative covalent, or a dative bond, which is indicated using an arrow.
Now that you’ve learned the principles of Lewis acids-and bases, let’s investigate whether a stable adduct will form between triphenylphosphine and borane.
Before you start, make sure you are familiar with the Schlenk Line and how to use it for solvent transfer. Wear appropriate PPE and inspect all glassware for star cracks.
Close the pressure release valve, turn on the N2 and vacuum pump. Assemble the cold trap and fill it with dry ice/acetone, once minimum pressure is reached. This way you minimize the risk of O2 condensation in the trap, which is explosive in presence of organic solvents.
Now, let’s start the synthesis by adding 5.3 g of triphenylphosphine to a 200 mL Schlenk flask labeled as A. Prepare Schlenk flask A for the cannula transfer of solvent.
Add 20 mL of dry and degassed THF to Schlenk flask A using cannula transfer. Stir the solution to dissolve triphenylphosphine. Meanwhile, prepare a second Schlenk flask B containing 1.15 g of NaBH4 for cannula transfer.
Cool both Schlenk flasks A and B in an ice bath. Using the cannula, transfer the contents of flask A into flask B. Next, replace the rubber septum of Schlenk B with an addition funnel, purge the funnel, and fit it with a new septum.
Next, add 8 mL of dry and degassed THF to the addition funnel via cannula transfer. With the system under N2, remove the septum from the addition funnel, add 2 mL of glacial acetic acid, and put the septum back on. Now, add the THF and glacial acid mixture drop wise to Schlenk flask B, while stirring vigorously.
After the addition, allow the reaction to warm up to room temperature and stir for an extra hour under N2. Then close the N2 supply, remove the addition funnel, and quench the reaction slowly with 20 mL of H2O.
Next, add a mixture of acetic acid in water slowly to the reaction, inducing product precipitation. Cool the flask, if no precipitate forms.
Filter the product by suction through a fritted funnel. Wash the resulting solid with 20 mL of ice cold water, and transfer the precipitate to a flask for drying.
Lastly, prepare an NMR sample of the starting material and the isolated product in CDCl3. Collect a 31P NMR for each sample.
Now let’s analyze how the phosphorous signal of triphenylphosphine is affected upon the coordination to borane in the product using the NMR.
Free triphenylphosphine shows as signal at -5.43 ppm, while the signal of the borane triphenylphosphine complex is shifted downfield to 20.7 ppm. This is consistent with the removal of electron density from the phosphorous center, which is deshielded upon Lewis adduct formation.
This observation reinforces the Lewis acid-base theory predicting that borane, as a Lewis acid, and triphenylphosphine, as a Lewis base, will form a stable adduct.
The Lewis acid-base model is used to gain more insight into molecular characteristics, which is necessary when designing new syntheses in organic and inorganic chemistry for molecules including transition metals.
Historically, transition metal ions have been regarded as Lewis acids, however, they can also serve as Lewis bases. For example, metal-borane complexes can participate in important transformations such as hydrogenation of olefins and nitrogen fixation.
Olefin hydrogenation can be performed using a new catalyst based on a nickel borane species. This species cleaves the H-H bond heterolytically and reversibly adds the H2 to the olefin transforming it to an alkane.
Furthermore, an iron-borane complex homogeneous catalyst can catalytically reduce nitrogen to ammonia, which is a critical reaction in the chemical industry.
Frustrated Lewis Pairs, or FLPs, are Lewis acid-base adducts, which cannot form a dative bond, due to steric hindrance.
The reactivity of frustrated Lewis pairs has found application in the development of new hydrogenation catalysts. For instance, it was shown that a zwitterionic complex, which is based on main group elements, reversibly loses H2 to give this product. This study pioneered the development of FLP research.
You’ve just watched JoVE’s introduction to the Lewis acid-base theory. You should now understand the definition of Lewis acids- and bases, how to synthesize a Lewis acid-base complex, and where these types of complexes are applied. Thanks for watching!
