Ozonólisis de alquenos
English
Diviser
Vue d'ensemble
Fuente: Vy M. Dong y Zhiwei Chen, Departamento de química, Universidad de California, Irvine, CA
Este experimento demuestra un ejemplo de una reacción de ozonólisis para sintetizar vainillina de isoeugenol (figura 1). Ozonólisis de alquenos, una reacción de oxidación entre el ozono y un alqueno, es un método común para preparar aldehídos, cetonas y ácidos carboxílicos. Este experimento también demuestra el uso de un generador de ozono y una reacción de baja temperatura (−78 ° C).
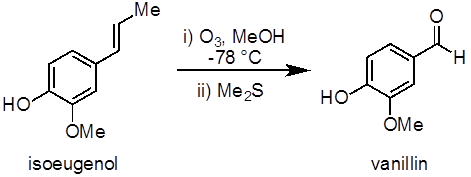
Figura 1. Diagrama que muestra la ozonólisis de isoeugenol a vainillina.
Principles
Procédure
Résultats
Vanillin was obtained as a white solid (150 mg, 76% yield); m.p. 76-79 °C; 1H NMR (400 MHz, CDCl3) δ 9.82 (br s, 1H), 7.43-7.41 (m, 2H), 7.04 (d, J = 8.8 Hz, 1H), 6.30 (s, 1H), 3.96 (s, 3H).
Applications and Summary
In this experiment, we have demonstrated the synthesis of vanillin from isoeugenol using the ozonolysis reaction. Also, using an ozone generator while performing a low temperature reaction was shown.
Ozonolysis is a useful reaction to prepare aldehydes, ketones, and carboxylic acids from alkenes. It has been applied in natural product synthesis and industrial-scale preparation of pharmaceuticals. Artemisinin is a potent antimalarial agent and was one of the natural products recognized in the 2015 Nobel Prize in Medicine. In a 10-step synthesis from (R)-(+)-pulegone, ozonolysis was used in the last step to make the natural product (Figure 5). Ceftibuten and cefaclor are cephalosporin antibiotics produced on industrial scale. One commercial route uses ozonolysis to access a common key intermediate, which can be elaborated to both compounds (Figure 6).
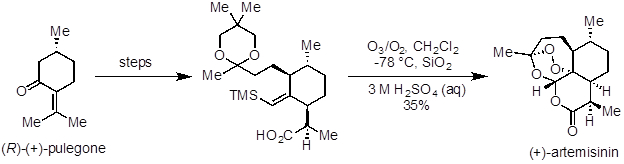
Figure 5. Diagram showing ozonolysis as the last step in a synthesis of artemisinin.
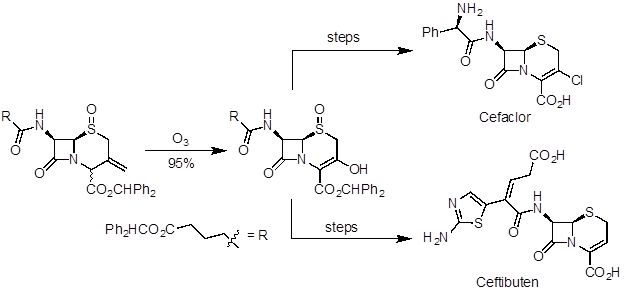
Figure 6. Diagram showing ozonolysis to prepare a key intermediate in the divergent synthesis of cefaclor and ceftibuten.
Transcription
Ozonolysis is the oxidation of unsaturated bonds in organic compounds by ozone.
Ozonolysis is most frequently used to cleave alkenes to obtain two carbonyl products. Ozone also reacts with alkynes and hydrazones. Ozonolysis is used in organic chemistry research, particularly natural product synthesis, and in industrial-scale synthesis of pharmaceuticals.
This video will illustrate the procedure for ozonolysis of alkenes and introduce a few applications of ozonolysis in chemistry.
Ozonolysis of an alkene begins with cycloaddition of ozone across the carbon-carbon double bond to form an unstable intermediate called a molozonide. The molozonide then dissociates into a carbonyl oxide and a carbonyl. These fragments rearrange to move the carbonyl oxygen away from the carbonyl oxide oxygens. The fragments then recombine by cycloaddition into a more stable ozonide, in which the carbonyl oxygen is bound to the carbon atoms.
The ozonide is worked up to cleave the ring into the carbonyl products. If both substituents on a side are carbon groups, a ketone is formed regardless of the workup conditions. Given a hydrogen substituent, oxidative workup with a hydrogen peroxide will produce a carboxylic acid. Reductive workup with zinc in acetic acid or dimethyl sulfide will produce an aldehyde.
Alkene ozonolysis is usually performed in the presence of an indicator to track the reaction progress. An indicator is a compound with a different visual appearance at different stages in the reaction. For example, ozonolysis of the indicator Sudan III results in a color change from bright red to deep blue or purple. Alkenes undergo ozonolysis faster than Sudan III, so the color change indicates that the alkene ozonolysis reaction has completed.
Now that you understand the principles of ozonolysis of alkenes, let’s go through a procedure for ozonolysis of isoeugenol in one of the pioneering reactions for commercial vanillin synthesis.
To begin the procedure, combine 200 mg of isoeugenol, 15 mL of methanol, and approximately 2 mg of the indicator Sudan III in a 3-necked round-bottom flask equipped with a stir bar.
Secure the flask in a fume hood over a stir plate. Ensure that the ozone generator is off, and then connect the flask to the generator and an oil bubbler.
Turn on the stirring motor to mix the solution. Ensure that the red indicator is evenly distributed throughout the reaction mixture. Then, prepare a cooling bath of dry ice in acetone.
Start the oxygen flow via the ozone generator. Place the cooling bath on a lab jack and raise the bath to cool the reaction mixture. Once the mixture has cooled, start the ozone generator.
Monitor the reaction mixture as it stirs until the mixture becomes blue to purple in appearance. Then, turn off the ozone generator and purge the reaction mixture with diatomic oxygen for 5 min.
Once the purging is complete, turn off the oxygen flow and lower the cooling bath out from under the flask. To begin the reductive workup, add 0.2 mL of dimethyl sulfide to the flask. Allow the mixture to warm to room temperature while stirring for 1 h.
Then, remove excess solvent with a rotary evaporator. Once this is done, dissolve the residue in 10 mL of 10% ethyl acetate in hexanes.
Connect a vacuum filtration flask to a vacuum line. Secure a Büchner funnel in the flask and load the funnel with silica gel.
Purify the product mixture by vacuum filtration. Wash the product through the silica gel with two portions of 10% ethyl acetate in hexanes.
Transfer the filtrate to a round-bottomed flask. Again remove the solvent with a rotary evaporator to obtain vanillin as yellow to white crystalline needles.
Calculate the percent yield, determine the melting point, and obtain a proton NMR spectrum of the product.
Vanillin is obtained as a white crystalline solid. The melting point range in literature is 81 to 83 degrees. Proton NMR spectra may be compared to previous literature reports.
Ozonolysis is widely used in chemistry and biology. Let’s look at a few examples.
Ozonolysis is a step of many industrial syntheses of pharmaceuticals. For instance, the antibiotics ceftibuten and cefaclor are produced from a common intermediate product accessed by ozonolysis of a terminal alkene to a ketone. The intermediate ketone product can interconvert with an enol form. The enol hydroxy group then undergoes different reactions in the remaining steps to the two antibiotics.
Ozonolysis can be the final step of a technique for stereoselectively alkylating the alpha carbon of aldehydes and ketones. In this reaction, a pyrrolidine reagent controls the stereoselectivity of the carbon-carbon bond formation by forming a sterically bulky hydrazone. After alkylation of the alpha carbon, the carbon-nitrogen double bond of the hydrazone can be cleaved by ozonolysis to regenerate the ketone or aldehyde following reductive workup.
You’ve just watched JoVE’s introduction to ozonolysis. You should now understand the underlying principles of ozonolysis, the procedure for ozonolysis of alkenes, and a few examples of how ozonolysis is used in organic chemistry. Thanks for watching!
