- 00:04Vue d'ensemble
- 00:51Principles of Palladium-Catalyzed Cross-Coupling Reactions
- 02:52Heck Coupling Reaction
- 03:52Isolation and Purification of Heck Coupling Product
- 04:46Applications
- 06:03Summary
Acoplamiento cruzado catalizado por paladio
English
Diviser
Vue d'ensemble
Fuente: Vy M. Dong y Faben Cruz, Departamento de química, Universidad de California, Irvine, CA
Este experimento demuestra el concepto de un acoplamiento cruzado catalizado por paladio. Se ilustrará la puesta en marcha de una reacción de acoplamiento cruzado catalizado por paladio típico. Reacciones de acoplamiento cruzado catalizadas por paladio han tenido un profundo efecto en químicos sintéticos cómo crear las moléculas. Estas reacciones han permitido químicos para la construcción de vínculos en formas nuevas y más eficientes. Tales reacciones han encontrado aplicaciones generalizados en las industrias química y farmacéuticas finas. Reacciones de acoplamiento cruzado catalizadas por paladio añadir otra herramienta a la caja de herramientas de la farmacia para la construcción de bonos de carbono, que son esenciales para la química orgánica. La combinación de la importancia de hacer bonos de carbono y el impacto de acoplamiento cruzado catalizadas por paladio han resultado de estas reacciones está el tema del Premio Nobel 2010 en química. EI-ichi Negishi, uno de los receptores del Premio Nobel 2010 en química, explicó en su conferencia Nobel que una de sus motivaciones para el desarrollo de esta química fue “ampliamente aplicable simple Lego-como métodos para conectar dos diferentes grupos orgánicos”.
Principles
Procédure
Résultats
The product should be a solid with the follow 1H NMR spectrum: 1H NMR (400 MHz, DMSO-d6): δ (ppm) 2.60 (s, 3H), 6.67 (d, J = 16.0 Hz, 1H), 7.65 (d, J = 16.0 Hz, 1H). 7.83 (d, J = 8.4 Hz, 2H). 7.97 (d, J = 8.4 Hz, 2H).
Applications and Summary
These Pd-catalyzed cross coupling reactions have changed the way molecules are synthesized in academic and industrial settings. The impact of this technology can be seen in how chemists construct complex structures for pharmaceuticals, agriculture chemicals, and materials. Beyond Pd-catalyzed cross couplings, transition metal catalysis has changed (and is continuing to change) the way synthetic chemists prepare molecules that can have an impact on society through their potential therapeutic use.
Many molecules of interest for treating diseases have linkages connecting aromatic or heteroaromatic rings. Palladium cross coupling reactions, like the Suzuki reaction, have found widespread use in the pharmaceutical industry for making these types of structures. For example, Crizotinib (Xalkori), an anti-cancer drug for the treatment of non-small cell lung carcinoma, is synthesized on a several kilo-scale using a Suzuki coupling.
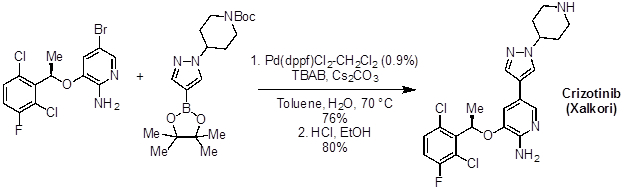
Figure 3: Crizotinib (Xalkori), an anti-cancer drug.
Palladium cross couplings have also been applied towards the synthesis of Taxol (an anticancer drug), Varenicline (an anti-smoking drug), and precursors for high performance electronic resins.
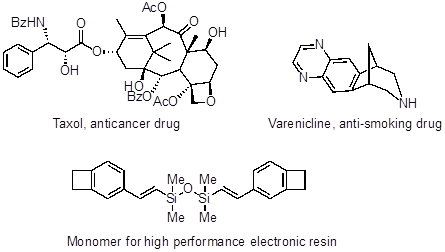
Figure 4: Taxol (an anticancer drug), Varenicline (an anti-smoking drug), and precursors for high performance electronic resins.
Transcription
Palladium-catalyzed cross-coupling reactions are a valuable tool for creating new carbon-carbon bonds.
Their development has enabled chemists to construct complex organic compounds from hydrocarbon fragments, and their use in the fine chemical and pharmaceutical industries has become wide-spread.
So profound is their impact on organic chemistry that they were the subject of the 2010 Nobel Prize in Chemistry.
This video will illustrate the principles of palladium-catalyzed cross coupling reactions, as well as a demonstration of the technique in the laboratory.
In these types of reactions, a palladium catalyst is used to facilitate the addition of a nucleophile, which is typically an organometallic compound, to a nucleophile, which is typically an organohalide
A Pd(0) species reacts with the organohalide via oxidative addition to form an organopalladium two species, which then reacts with the nucleophile to form a new carbon-carbon bond.
All of these reactions, with the exception of Heck coupling, follow the same mechanism: following the oxidative addition to form an organopalladium two species, a transmetallation step with the organometallic nucleophile occurs, forming a new species with two carbon-palladium bonds. Finally, reductive elimination occurs to create a new carbon-carbon bond and to regenerate palladium zero catalyst, which can continue to couple another nucleophile and electrophile.
In Heck coupling reactions, the organopalladium two species is formed similarly to the previous coupling reactions, but as opposed to a transmetallation step, a coordination step occurs in which the palladium two species forms a complex with an olefin. Following the coordination step, carbopalladation occurs to generate a new carbon-carbon and carbon-palladium bond. Next, beta-hydride elimination yields the desired substituted olefin and a palladium two hydride species, which undergoes reductive elimination to regenerate the palladium zero catalyst.
Now that we have discussed the principles of palladium-catalyzed cross-coupling reactions, let’s look at a typical Heck coupling procedure.
To synthesize an α,β-unsaturated carboxylic acid, we will react 4-iodoacetophenone and acrylic acid in the presence of a palladium catalyst. Begin by filling a 20-mL round-bottomed flask with acetophenone, acrylic acid, palladium two chloride, and dilute with 5 mL of water. Then add sodium carbonate to reduce the catalyst to palladium zero, and add a magnetic stir bar for mixing
After adding the reagents to the flask, stir the contents, and heat the reaction to reflux
Monitor the progress of the reaction by thin layer chromatography, or TLC, ensuring the complete consumption of acetophenone.
Once the reaction is complete, remove the flask from the heating source, and allow the mixture to cool to room temperature.
Once the mixture has cooled, acidify to pH 1 with 1 molar aqueous HCl, monitoring the pH with litmus paper, and the coupling product should precipitate from the solution.
After the mixture has been acidified, pour the contents onto a Büchner funnel covered with filter paper, and collect the solid by vacuum filtration.
To verify the structure of the coupling product, dissolve 2 mg of the dried material in 0.5 mL DMSO-d6 and analyze by NMR.
Now that we have seen an example laboratory procedure, let’s see some useful applications of palladium-catalyzed cross-coupling reactions.
A major requirement in the manufacture of pharmaceutical compounds is minimal toxicity and flammability, and maximum stability in the process. Suzuki coupling reaction conditions are fairly safe, and this reaction is widely used in process chemistry, as in the synthesis of crizotinib, a lung cancer drug, from an aryl bromide and a boronic ester.
Taxol, a natural product also with anticancer properties, was discovered in the bark of the Pacific yew tree, Taxus brevifolia. Unfortunately, only 10 grams of pure compound can be collected per 1.2 tons of bark. The quantity of taxol needed in the clinic required the development of an efficient chemical synthesis, and an intramolecular Heck coupling reaction was instrumental in its large-scale production.
You’ve just watched JoVE’s introduction to palladium-catalyzed cross-coupling reactions. You should now understand the principles behind them, how to perform an experiment, and some of their uses. Thanks for watching!
