Application of Elemental Lanthanides in the Selective C-F Activation of Trifluoromethylated Benzofulvenes Providing Access to Various Difluoroalkenes
PRÉPARATION DE L'INSTRUCTEUR
concepts
PROTOCOLE ETUDIANT
1. Preparation of Starting Material Outside the Glovebox
- Prepare necessary equipment: oven-dried Schlenk tube with a magnetic stir bar, vacuum/inert gas line (argon or nitrogen), and magnetic stirrer.
- Prepare trifluoromethylated benzofulvene according to the literature13.
- Weigh out trifluoromethylated benzofulvene (177 mg, 0.65 mmol) and add into an oven-dried Schlenk tube equipped with a magnetic stir bar.
- Close the Schlenk tube with a plastic screw cap with PTFE liner and connect to vacuum line.
- Start stirring and apply vacuum to dry benzofulvene for 10 min. Then, release the vacuum with argon/nitrogen and again apply the vacuum. Repeat this process 3 times. Finally, fill the Schlenk tube with argon/nitrogen and close it tightly.
- Take the Schlenk tube under argon/nitrogen to the glove box.
2. Preparation of Freshly Filed Dysprosium Metal Inside the Glovebox
- Prepare necessary equipment: glove box under argon/nitrogen with weighing balance, metal file, pliers, aluminum foil, forceps, spatula, test tube, and 2 rubber septa.
- Prepare chemicals: dysprosium metal and anhydrous AlCl3
- Insert Schlenk tube with benzofulvene, a test tube and 2 rubber septa into the glovebox according to your group's general practice for handling the glovebox, e.g., for a small chamber apply vacuum for 1 min then release with argon/nitrogen three times.
- Put one piece of aluminum foil onto the weighing balance and one onto a plate on the glovebox floor for filing.
- Tightly hold the dysprosium metal with the help of pliers and remove its upper inactive layer by filing with a metal file to obtain a shiny metal surface. Discard this filed metal.
NOTE: (1) Use large metal pieces for filing and avoid contact between the pliers and the file in order not to contaminate the dysprosium metal with other metals. (2) Use a different metal file for each lanthanide metal to avoid cross-contamination. (3) Discarded metal powder should be quenched separately outside the glove box, using dil. aq. HCl solution.
Caution: The reaction of dysprosium with aq. HCl is exothermic and produces hydrogen gas, therefore quench the dysprosium in an open flask surrounded by ice bath. - Prepare freshly filed dysprosium metal (82 mg, 0.50 mmol), add to the Schlenk tube, and close the tube with a rubber septum.
- Weigh anhydrous AlCl3 (200 mg, 1.5 mmol) into a test tube and close the tube with a rubber septum.
- Remove the Schlenk tube and the test tube from the glove box.
3. Start C-F Activation Reaction Outside the Glovebox
- Prepare necessary equipment: vacuum/inert gas line (argon or nitrogen), magnetic stirrer, and dry 2 mL syringe with needle.
- Prepare chemicals: freshly distilled dry THF and iodine.
- Clamp the Schlenk tube in a fume hood above a magnetic stirrer and connect to the vacuum line. Perform three cycles of vacuum/inert gas before opening the Schlenk tube under a positive flow of argon/nitrogen. Start the magnetic stirrer.
- Clamp the test tube with AlCl3 in a fume hood and connect to the inert gas line via a needle under a positive flow of argon/nitrogen.
- Add a catalytic amount of iodine (10 – 12 mg) to the Schlenk tube under a positive argon/nitrogen flow.
- Purge a dry 2 mL syringe with needle under argon/nitrogen
- Take 1.5 mL of freshly distilled dry THF using the purged syringe and add 0.5 mL to the Schlenk tube to obtain a deep brown solution (color of iodine in THF).
- Add the remaining 1.0 mL of THF dropwise to the test tube with AlCl3, which will lead to a slightly yellow solution.
NOTE: Depending on the quality of AlCl3, the addition of THF can be more or less exothermic. - While still warm, transfer this AlCl3 solution from the test tube to the Schlenk tube using the syringe.
- Allow the reaction to stir until the full consumption of benzofulvene. Initially, the deep brown color will disappear to give a yellow solution, which over time will turn to a dark green turbid solution.
- Check the reaction by thin-layer chromatography (TLC) after 1 h by taking a sample of the reaction mixture with a capillary under a positive flow of argon/nitrogen. Use petroleum ether as eluent.
NOTE: The starting benzofulvene is bright yellow on the TLC plate and its disappearance can be readily observed.
4. Addition of Nitroalkene
- Prepare 4-methoxyphenylnitroalkene according to the literature16.
- In a test tube weigh out 4-methoxyphenylnitroalkene (90 mg, 0.50 mmol), close the tube with a rubber stopper, and dry it on the vacuum line via a needle connection.
- After disappearance of benzofulvene in the reaction mixture (confirmed by TLC analysis), add dry nitroalkene to the Schlenk tube under a positive flow of argon/nitrogen. The reaction mixture will change color to yellow-green.
- Monitor the reaction by TLC analysis, until full consumption of nitroalkene (usually 1 hour). Use petroleum ether/ethyl acetate 95/5 as eluent.
5. Workup and purification
- Dilute the reaction mixture with 20 mL of diethyl ether and quench it with 5 mL of 1 molar aqueous solution of hydrochloric acid in an open Schlenk tube.
CAUTION: The reaction of remaining dysprosium with aq. HCl is exothermic and produces hydrogen gas, which may cause pressure build up in Schlenk. Therefore, quench the reaction in open Schlenk under a good fume hood. - After quenching the reaction, transfer it to a separatory funnel.
- Collect the organic phase in a conical flask. Wash the aqueous phase 2 times with 10 mL of diethyl ether and collect the organic phase in the conical flask.
- Dry the organic phase over MgSO4, filter into a pre-weighed flask, and concentrate under reduced pressure on a rotary evaporator to get the crude product as an orange-brown oil.
- Purify the crude product by silica gel column chromatography starting with petroleum ether as eluent to remove non-polar hydrolyzed product. Increasing the polarity up to 10/1 petroleum ether/ethyl acetate gives the new nitro-containing difluoroalkenes.
NOTE: When performing the column chromatography on a long column and by slowly increasing the polarity, it is possible to separate the diastereoisomers for analysis purposes.
Application of Elemental Lanthanides in the Selective C-F Activation of Trifluoromethylated Benzofulvenes Providing Access to Various Difluoroalkenes
Learning Objectives
This lanthanide-mediated C-F activation procedure followed by reaction with nitroalkenes provides readily access to new difluoroalkenes containing a nitro group. A plausible reaction mechanism is depicted in Figure 2. In contrast to our previous work using aldehydes as electrophiles (Figure 1)13, nitroalkenes afford the 1,3-disubstituted indene products regioselectively. This may be explained by the greater steric bulk of the nitroalkene group.
In order to find the best reaction conditions we investigated the combination of two different lanthanide metals (La, Dy) with AlCl3 in this reaction with nitroalkenes. It turned out that in both cases, the reaction proceeded with comparable regioselectivity and with the same poor diastereoselectivity. In the case of Dy, the yield was considerably higher than with La metal and we continued therefore our study with the Dy/AlCl3 system, as in our previous report with aldehydes13.
Under optimal conditions, the crude 19F NMR spectrum of the new difluoroalkene products in CDCl3 shows two sets of two doublets, one at -82.9 ppm and -86.7 ppm (J = 27 Hz) and the other one at -82.9 ppm and -86.8 ppm (J = 27 Hz), corresponding to the difluoroalkene groups of the two diastereoisomeric products. As an excess of starting benzofulvene is used, one will also observe another set of two doublets at -83.5 and -87.7 ppm (J = 28.2 Hz) corresponding to the hydrolyzed product, in variable yield (Figure 3).
In case moisture entered the reaction, e.g.,from starting materials or during the solvent/reagent transfers, or if the second step did not go to completion, the set of signals for the hydrolyzed product would increase. If the first step of the reaction did not go to completion, e.g., if the lanthanide metal was not reactive enough, some starting benzofulvene may still be observed at -56.3 ppm (Figure 3). 1H and 13C NMR spectroscopic analyses were in agreement with this description (Figure 4).
Analysis of the infrared spectrum of the final product also nicely shows the incorporation of the nitro group (1553 and 1375 cm-1) and the transformation of the CF3 group into the CF2 alkene (1707 cm-1). Analysis by high-resolution electron-impact mass spectrometry further confirmed the identity of the product, showing the parent ion at m/z = 433.1479 (calculated 433.1489).
This reaction works smoothly with a range of 2-arylnitroalkenes leading to the corresponding difluoroalkenes as 1:1 mixtures of diastereoisomers in good yields (Figure 5, Table 1). The aryl groups can contain an electron-donating group (-OMe) or electron-withdrawing groups (-Cl, Br) in the para-position. The reaction also proceeds with phenyl- or 1-naphthyl-containing nitroalkenes. We have previously shown that benzofulvenes substituted with aryl groups other than phenyl on the exocyclic carbon also afford a C-F activation reaction13. Here we include another example of 2-thienyl-substituted benzofulvene, which after C-F activation and reaction with p-Cl-phenylnitroalkene yields the corresponding difluoroalkene (entry 6). It should be noted that with the electron-rich p-dimethylaminophenyl group on the benzofulvene, no reaction occurs under these conditions13.
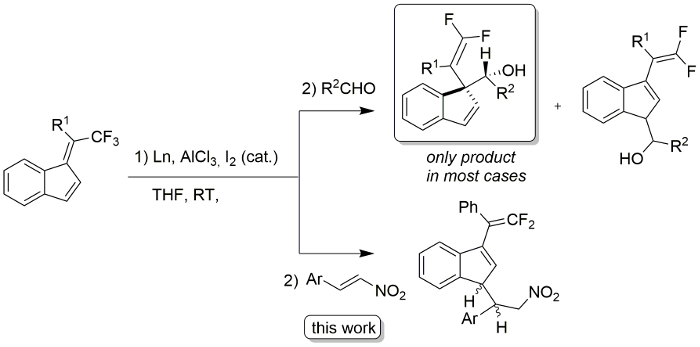
Figure 1: Lanthanide-mediated C-F activation in trifluoromethylated benzofulvenes. Please click here to view a larger version of this figure.
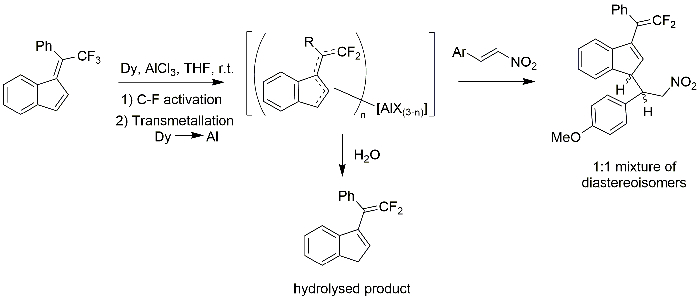
Figure 2: Plausible reaction mechanism for the formation of nitro-group containing difluoroalkenes via C-F activation. Please click here to view a larger version of this figure.
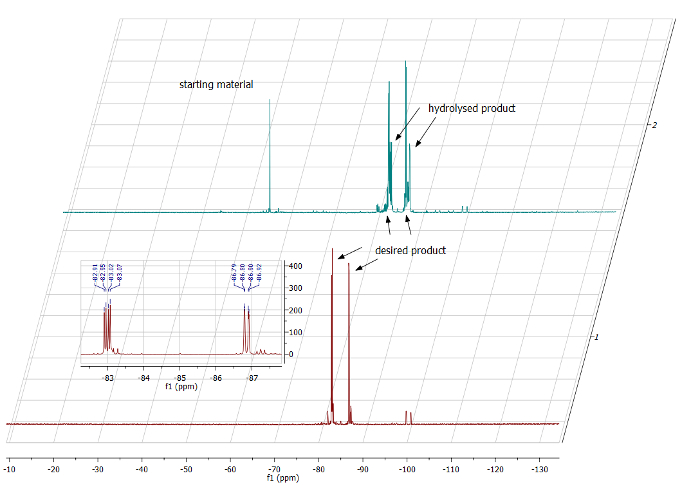
Figure 3: Comparison of crude 19F NMR spectra of two different reactions. Below, red: reaction worked well (insert shows the mixture of two diastereoisomers in a 1:1 ratio). Above, blue: reaction did not work well, with considerable amounts of starting benzofulvene and hydrolysed product present. Please click here to view a larger version of this figure.
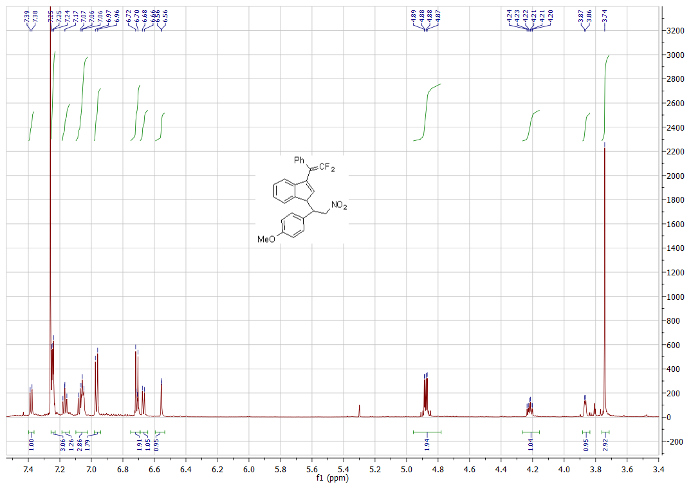
Figure 4: 1H NMR spectrum (600 MHz, CDCl3) of one diastereoisomer of nitro-containing difluoroalkene. Please click here to view a larger version of this figure.
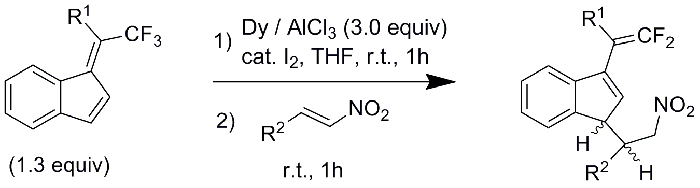
Figure 5: Scope of reaction with various 2-arylnitroalkenes Please click here to view a larger version of this figure.
| Entry | R1 | R2 | Yield (dr) |
| 1 | phenyl | phenyl | 72% (1:1) |
| 2 | phenyl | 4-methoxyphenyl | 78% (1:1) |
| 3 | phenyl | 4-chlorophenyl | 65% (1:1) |
| 4 | phenyl | 4-bromophenyl | 75% (1:1) |
| 5 | phenyl | 1-naphthyl | 62% (1:1) |
| 6 | 2-thienyl | 4-chlorophenyl | 55% (1:1) |
Table 1: Scope of reaction with various 2-arylnitroalkenes
List of Materials
| Dysprosium ingot | Strem | 93-6637 | Store under nitrogen/argon |
| Anhydrous aluminum chloride | Alfa Aesar | 88488 | Store under nitrogen/argon |
| Iodine 99.5% for analysis | Across Organics | 212491000 | |
| THF GPR Rectapur | VWR Chemicals | 28552.324 | Dried and distilled over Na/benzophenone before use |
| Glovebox | MBraun | Under nitrogen atmosphere |
Lab Prep
The selective activation of one carbon-fluorine bond in polyfluorinated aromatic molecules or in trifluoromethyl-containing substrates offers the possibility of accessing unique fluorine-containing molecules, which are difficult to obtain by other synthetic pathways. Among various metals, which can undergo C-F activation, lanthanides (Ln) are good candidates as they form strong Ln-F bonds. Lanthanide metals are strong reducing agents with a redox potential Ln3+/Ln of approximately -2.3 V, which is comparable to the value of the Mg2+/Mg redox couple. In addition, lanthanide metals display a promising functional group tolerance and their reactivity can vary along the lanthanide series, making them suitable reagents for fine-tuning reaction conditions in organic and organometallic transformations. However, due to their oxophilicity, lanthanides react readily with oxygen and water and therefore require special conditions for storage, handling, preparation, and activation. These factors have limited a more widespread use in organic synthesis. We herein present how dysprosium metal – and by analogy all lanthanide metals – can be freshly prepared under anhydrous conditions using glovebox and Schlenk techniques. The freshly filed metal, in combination with aluminum chloride, initiates the selective C-F activation in trifluoromethylated benzofulvenes. The resulting reaction intermediates react with nitroalkenes to obtain a new family of difluoroalkenes.
The selective activation of one carbon-fluorine bond in polyfluorinated aromatic molecules or in trifluoromethyl-containing substrates offers the possibility of accessing unique fluorine-containing molecules, which are difficult to obtain by other synthetic pathways. Among various metals, which can undergo C-F activation, lanthanides (Ln) are good candidates as they form strong Ln-F bonds. Lanthanide metals are strong reducing agents with a redox potential Ln3+/Ln of approximately -2.3 V, which is comparable to the value of the Mg2+/Mg redox couple. In addition, lanthanide metals display a promising functional group tolerance and their reactivity can vary along the lanthanide series, making them suitable reagents for fine-tuning reaction conditions in organic and organometallic transformations. However, due to their oxophilicity, lanthanides react readily with oxygen and water and therefore require special conditions for storage, handling, preparation, and activation. These factors have limited a more widespread use in organic synthesis. We herein present how dysprosium metal – and by analogy all lanthanide metals – can be freshly prepared under anhydrous conditions using glovebox and Schlenk techniques. The freshly filed metal, in combination with aluminum chloride, initiates the selective C-F activation in trifluoromethylated benzofulvenes. The resulting reaction intermediates react with nitroalkenes to obtain a new family of difluoroalkenes.
Procédure
The selective activation of one carbon-fluorine bond in polyfluorinated aromatic molecules or in trifluoromethyl-containing substrates offers the possibility of accessing unique fluorine-containing molecules, which are difficult to obtain by other synthetic pathways. Among various metals, which can undergo C-F activation, lanthanides (Ln) are good candidates as they form strong Ln-F bonds. Lanthanide metals are strong reducing agents with a redox potential Ln3+/Ln of approximately -2.3 V, which is comparable to the value of the Mg2+/Mg redox couple. In addition, lanthanide metals display a promising functional group tolerance and their reactivity can vary along the lanthanide series, making them suitable reagents for fine-tuning reaction conditions in organic and organometallic transformations. However, due to their oxophilicity, lanthanides react readily with oxygen and water and therefore require special conditions for storage, handling, preparation, and activation. These factors have limited a more widespread use in organic synthesis. We herein present how dysprosium metal – and by analogy all lanthanide metals – can be freshly prepared under anhydrous conditions using glovebox and Schlenk techniques. The freshly filed metal, in combination with aluminum chloride, initiates the selective C-F activation in trifluoromethylated benzofulvenes. The resulting reaction intermediates react with nitroalkenes to obtain a new family of difluoroalkenes.
