Human Serum Anti-aquaporin-4 Immunoglobulin G Detection by Cell-based Assay
PRÉPARATION DE L'INSTRUCTEUR
concepts
PROTOCOLE ETUDIANT
This procedure was approved by the Ethics Committee of the First Hospital of Jilin University and was performed on approximately 1,500 subjects.
1. Patient Enrollment and Blood Sample Collection
- Apply the laboratory detection of serum anti-AQP4 IgG to clinic patients with the chief complaints and symptoms listed below. Perform physical examinations as well.
Optic neuritis: Patients suffer with visual deficits, such as loss of visual fields and reduction of visual acuity.
Acute myelitis: Patients may present with paralysis, sensory deficits and autonomic dysfunction.
Area postrema syndrome: Patients may have symptoms of unexplained hiccups, nausea or vomiting.
Acute brainstem syndrome: Brainstem lesions could result in different symptoms and physical signs depending on the location of the lesions.
Symptomatic narcolepsy or acute diencephalic clinical syndrome with NMOSD-typical diencephalic MRI lesions.
Symptomatic cerebral syndrome with NMOSD-typical brain lesions.
NOTE: According to the international consensus diagnostic criteria for NMOSD5, a diagnosis of NMOSD can be made if the patient is serum anti-AQP4 IgG-positive and has at least one of the core clinical characteristics mentioned above. However, we do not recommend testing of anti-AQP4 IgG in patients with optic neuritis only. - Blood sample collection
NOTE: Patients do not need to be fasting.- Draw 2-3 mL of venous blood in a 4 mL vacuum blood collection tube.
- Allow the serum to clot for 30 min at room temperature (RT).
NOTE: Prolonged clotting can increase serum levels due to leakage from platelets. - Centrifuge at 2100 x g for 5 min. Carefully transfer the serum to a new tube.
- Analyze the serum immediately or aliquot and store at -20 or -80 °C.
NOTE: Storage at -20 °C is for storage of months, while -80 °C is for storage of years.
2. Anti-AQP4 Antibody Detection
CAUTION: Patient samples and used kit reagents should be considered infectious materials. Sodium azide-containing reagents in the kit are toxic. A flow chart of the protocol is provided in Figure 1.
- Preparation
- Bring the kit to RT before use.
NOTE: Store the kit at 2-8 °C. - Prepare at least 200 mL of PBS wash buffer containing 0.2% Tween 20. Pour 100 mL of wash buffer into a 500 mL beaker.
- Dilute the serum samples 10x with wash buffer. Prepare the positive and negative controls according to the instructions of the distributor.
- Bring the kit to RT before use.
- Sample incubation
- Add 30 µL of the pre-diluted samples, positive control, or negative control to the reaction fields of the reagent tray (Figure 2).
NOTE: Avoid air bubbles. - Remove the protective cover on the biochip slides.
NOTE: Do not touch the biochips to avoid the detachment and contamination of fixed cells. Hold the slide side-by-side before removing the cover. - Apply the biochip slide to the reagent tray (Figure 2). Start a 30 min incubation at RT.
NOTE: Every sample should be an individual droplet connected to the corresponding biochip without mixing with other droplets. - Gently rinse the biochip slide once with wash buffer. Immerse the biochip slide into the 500 mL beaker filled with 100 mL of wash buffer for at least 5 min. Place the beaker on a shaker if possible.
- Take out the biochip slide from the wash buffer. Carefully wipe away the residual wash buffer outside of the reaction fields with a paper towel. Expose the biochip slide in open air for 1-2 min to evaporate the residual wash buffer on the reaction field.
NOTE: Do not dry out the reaction fields. Do not touch the biochips with paper towel.
- Add 30 µL of the pre-diluted samples, positive control, or negative control to the reaction fields of the reagent tray (Figure 2).
- Secondary antibody incubation
- Prepare the secondary antibody solution according to the instructions of the distributor.
- Add 25 µL of fluorescent secondary antibody to the reaction fields of a clean reagent tray.
- Apply the biochip slide to the reagent tray loaded with secondary antibody. Incubate for 30 min at RT.
- Mounting
- Pour out the used wash buffer and add 100 mL of fresh wash buffer into the beaker. Repeat the washing as described in steps 2.2.4 and 2.2.5.
- Carefully add embedding medium to the reaction fields on a biochip slide (one drop per reaction field). Seal the biochip slide with one piece of cover glass. Avoid air bubbles.
- Fluorescence detection
- Turn on the fluorescent lamp of microscope and pre-heat for 15 min. Choose a 488 nm filter.
- Open photograph software. Take pictures from both transfected and untransfected areas of all reaction fields with different magnifications. First, take one picture with 4x magnification for an overview, then take more pictures with 10x and 20x magnifications.
NOTE: The detailed protocol is shown in Table 1. Interpretation of the results is discussed in the representative results section.
3. Diagnosis of Patients
- If the patient is serum anti-AQP4 IgG-positive and shows at least one core clinical characteristic of NMOSD (see discussion section), diagnose the patient with NMSOD5. However, if the patient is anti-AQP4 IgG-negative, a diagnosis of NMOSD may not be excluded.
Human Serum Anti-aquaporin-4 Immunoglobulin G Detection by Cell-based Assay
Learning Objectives
Using the procedure described here, specific anti-AQP4 IgG in serum is detectable. During the procedure, pre-diluted samples, a positive control, and a negative control were added to the reaction fields, which contain transfected and untransfected areas (Figure 2). Fluorescence of the negative control in a transfected area mainly indicated the unspecific binding of secondary antibody to the transfected cells on biochips (Figure 3). Homogeneously weak fluorescence was visible (Figure 3). As for the positive control, anti-AQP4 antibody was added to the reaction field. Strong fluorescence was observed in the transfected area, which indicated the specific binding of anti-AQP4 IgG to AQP4-M1 in the transfected cells (Figure 4). Fluorescence of the positive control in an untransfected area showed hints of unspecific binding of anti-AQP4 IgG to fixed cells on biochips (Figure 4). For 10x diluted samples, anti-AQP4 IgG-negative serum showed a fluorescent pattern similar to the negative control, while the positive serum showed a pattern similar to the positive control (Figure 5). The intensity of fluorescence was associated with the anti-AQP4 IgG titer. Examples of different intensity of fluorescence are presented in Figure 5. Figure 5A and 5B were from anti-AQP4 IgG-negative samples, while Figure 5C-H were from anti-AQP4 IgG-positive samples. As long as heterogeneous and granular fluorescence appeared in transfected areas, the sample was considered anti-AQP4 IgG-positive, regardless of the strength of the fluorescence. The diagnostic criteria for NMOSD, which is based on the serum anti-AQP4 IgG, will be further described in the discussion section.
In a few samples, only a small number of cells was strongly stained in transfected areas, and it was hard to determine whether the serum was anti-AQP4 IgG-positive or negative (Figure 6). In this case, "probable positive" can be reported. In rarer cases, the fluorescence of a sample in transfected areas was homogeneously stronger than in untransfected areas (Figure 7). In this case, the sample should be regarded as anti-AQP4 IgG-negative. The high intensity of background fluorescence might be nonspecific. To create a reliable report, all photographs should be blindly evaluated by at least two clinicians. The clinicians should be trained before evaluation. In this protocol, they were shown representative photographs of different sample types and were informed as to how to evaluate results. Then, they evaluated photographs together with an experienced clinician. Finally, the trained clinicians worked independently. In an ideal situation, it should be always the same clinicians who evaluate results.
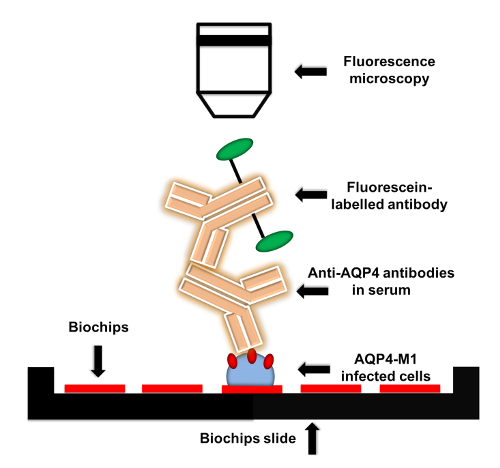
Figure 1: Flow chart of anti-AQP4 IgG detection protocol. AQP4-M1-transfected or untransfected EU 90 cells are fixed on the biochips. When adding serum to biochips, anti-AQP4 IgG in serum is captured by the fixed transfected cells. Then, fluorescein-labelled secondary antibody is applied to detect anti-AQP4 IgG. The fluorescence can be visualized by microscopy with various magnifications. Please click here to view a larger version of this figure.
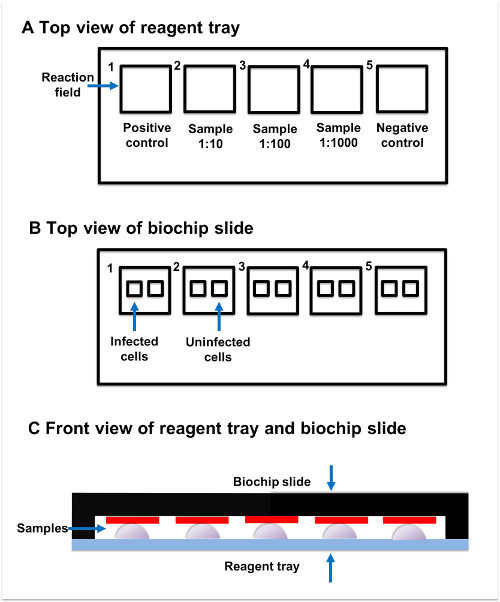
Figure 2: Sample incubation. (A) Top view of reagent tray. Every reagent tray contains five individual reaction fields. The positive control, samples, and negative control should be added to the separate reaction fields. (B) Top view of biochip slide. Every biochip slide contains five reaction fields, which have two subsections. The transfected subsection contains fixed AQP4-M1-transfected cells, while the untransfected subsection contains untransfected cells. (C) Front view of reagent tray and biochip slide. Reaction fields on the reagent tray and biochip slide are paired with each other. The sample added to the reaction field on reagent tray should be connected to the corresponding reaction field on the biochip slide. Please click here to view a larger version of this figure.
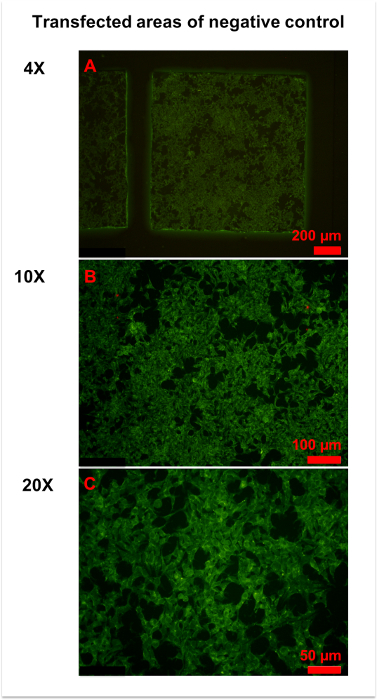
Figure 3: Representative figures for negative control. (A) An overview of the transfected area of the negative control was obtained by 4x magnification microscopy. Homogeneously weak fluorescence was observed throughout the area. (B,C) More details were observed in 10x (B) and x (C) magnification photographs. Generally, the cell membrane and plasma were slightly stained, and the cell nucleus was unstained. Please click here to view a larger version of this figure.
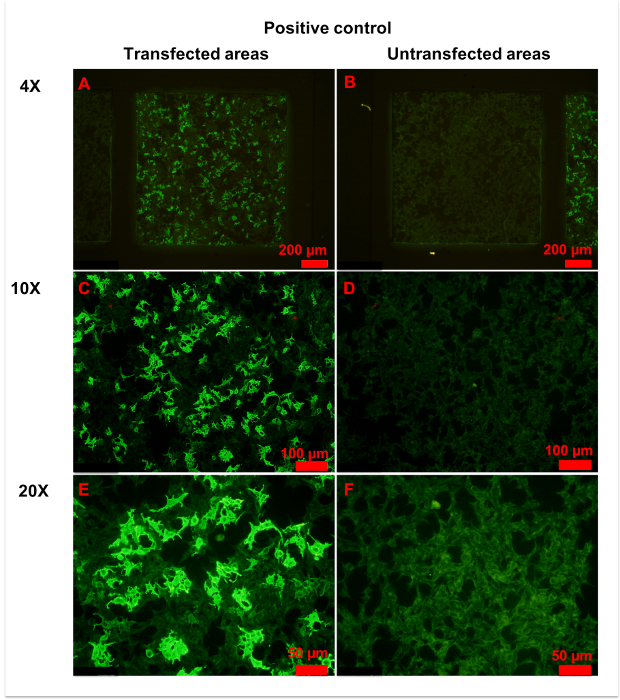
Figure 4: Representative figures for positive control. (A,B) Transfected (A) and untransfected (B) areas of positive control are shown. Homogeneously weak fluorescence was observed in untransfected areas, indicating unspecific binding of anti-AQP4 IgG to fixed cells. Conversely, heterogeneous strong fluorescence was observed in transfected areas, indicating specific binding of anti-AQP4 IgG to AQP4-M1 expressed on fixed cells. (C-F) More details of transfected (C,E) and untransfected (D,F) areas are observed in with 10x (C, D) or 20x (E,F) magnification. Weak and even fluorescence appeared in untransfected areas. However, a large percentage of cells in transfected areas showed intense fluorescence. In the plasma of strongly stained cells, anti-AQP4 IgG showed smooth and granular fluorescence. The cell nucleus was unstained or slightly stained. Please click here to view a larger version of this figure.
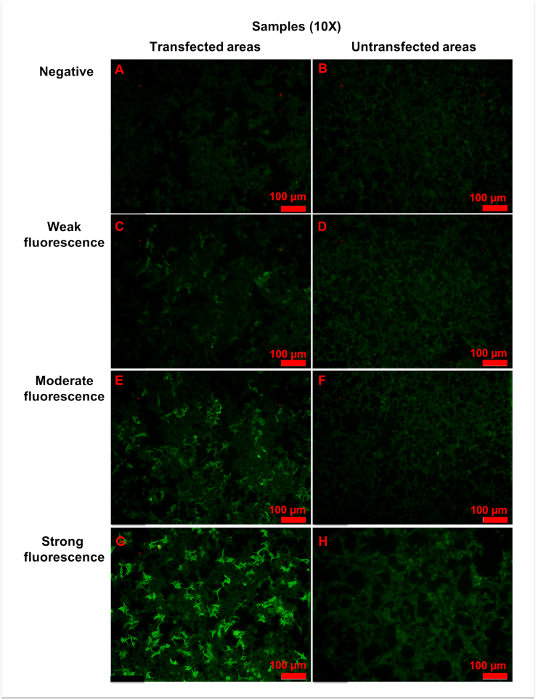
Figure 5: Representative figures for anti-AQP4 antibody negative or positive samples. Samples were 10x diluted, and all figures shown were taken with a 10x objective. (A,B) Negative fluorescence sample: the fluorescence was homogeneously weak in both (A) transfected and (B) untransfected areas. (C,D) Weak fluorescence sample: when compared with untransfected areas (D), a small amount of cells (approximately 25% to 50%) in transfected areas (C) showed more intense fluorescence. (E,F) Moderate fluorescence sample: in transfected areas (E), strong granular fluorescence was observed in a medium amount of cells (approximately 50% to 75%). (G,H) Strong fluorescence sample: cells in untransfected areas (H) were weakly stained. However, in transfected areas (G), granular fluorescence with high intensity appeared in a large number of cells (approximately over 75%). Generally, as the titer of anti-AQP4 IgG increased, both fluorescence intensity and percentage of strongly stained cells elevated correspondingly. The fluorescence of untransfected areas was always homogeneously weak. Please click here to view a larger version of this figure.
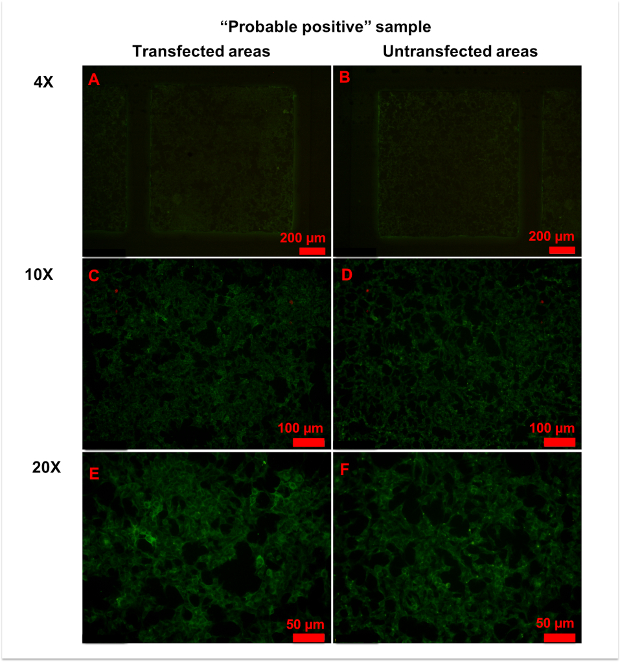
Figure 6: Representative figures for anti-AQP4 antibody "probable positive" samples. (A,B) No significant differences in fluorescence patterns were observed between transfected (A) and untransfected (B) areas at 4x magnification. (C-E) With 10x (C,D) or 20x (E,F) magnification, a few cells in transfected areas (C,E) showed strong granular fluorescence. Please click here to view a larger version of this figure.
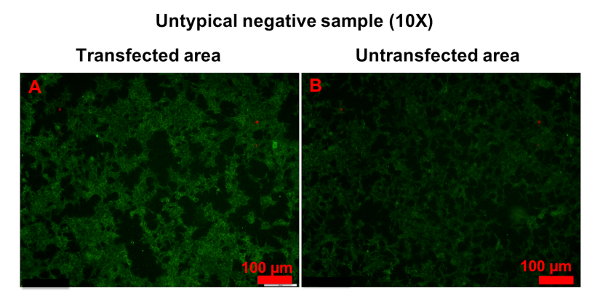
Figure 7: Example of untypical anti-AQP4 IgG-negative sample. Both transfected (A) and untransfected (B) areas were homogeneously stained. However, the fluorescence in transfected areas was stronger than in untransfected areas. Please click here to view a larger version of this figure.
| Magnification | Transfected area | Untransfected area |
| 4x | 1 picture | 1 picture |
| 10x | 4 pictures | 1 picture |
| 20x | 5 pictures | 1 picture |
Table 1: Fluorescence microscopy. It is recommended to use 4x, 10x, and 20x objectives for imaging of transfected and untransfected areas.
List of Materials
| Anti-aquaporin-4 IIFT | Euroimmun | FA 1128-2005-50 | Contains biochip slides coated with AQP4-M1 transfected and untransfected EU 90 cells, fluorescein-labelled anti-human IgG, anti-AQP4 antibody as positive control, antibody negative sample, salt for PBS pH 7.2, Tween 20 and embedding medium. |
| CellSens Dimension | OLYMPUS | N/A | photograph software |
| Gel & clot activator tube | Improve medical | 623040202 | From a local Chinese company |
Lab Prep
Anti-aquaporin-4 (AQP4) immunoglobulin G (IgG) is the core diagnostic biomarker for neuromyelitis optica spectrum disorders (NMOSD). The cell-based assay (CBA) is a widely used method to detect anti-AQP4 IgG in human serum with high sensitivity and specificity. Briefly, serum anti-AQP4 IgG is captured by AQP4-transfected cell that is fixed on the biochip then detected by a fluorescein-labelled secondary antibody. Fluorescence microscopy is utilized to visualize the fluorescence, and the intensity of fluorescence is evaluated by at least two experienced clinicians. A final diagnosis of NMOSD can be made based on the combination of anti-AQP4 IgG detection results, clinical manifestations, and neuroradiological findings. According to previous studies, CBA is more sensitive and specific than other anti-AQP4 IgG detection methods, and it can be applied to both clinical diagnosis and studies of NMOSD. The method has limitations; for example, an international scale to evaluate serum anti-AQP4 IgG titers is still lacking. Here, a detailed protocol for human serum anti-AQP4 IgG detection using CBA is described.
Anti-aquaporin-4 (AQP4) immunoglobulin G (IgG) is the core diagnostic biomarker for neuromyelitis optica spectrum disorders (NMOSD). The cell-based assay (CBA) is a widely used method to detect anti-AQP4 IgG in human serum with high sensitivity and specificity. Briefly, serum anti-AQP4 IgG is captured by AQP4-transfected cell that is fixed on the biochip then detected by a fluorescein-labelled secondary antibody. Fluorescence microscopy is utilized to visualize the fluorescence, and the intensity of fluorescence is evaluated by at least two experienced clinicians. A final diagnosis of NMOSD can be made based on the combination of anti-AQP4 IgG detection results, clinical manifestations, and neuroradiological findings. According to previous studies, CBA is more sensitive and specific than other anti-AQP4 IgG detection methods, and it can be applied to both clinical diagnosis and studies of NMOSD. The method has limitations; for example, an international scale to evaluate serum anti-AQP4 IgG titers is still lacking. Here, a detailed protocol for human serum anti-AQP4 IgG detection using CBA is described.
Procédure
Anti-aquaporin-4 (AQP4) immunoglobulin G (IgG) is the core diagnostic biomarker for neuromyelitis optica spectrum disorders (NMOSD). The cell-based assay (CBA) is a widely used method to detect anti-AQP4 IgG in human serum with high sensitivity and specificity. Briefly, serum anti-AQP4 IgG is captured by AQP4-transfected cell that is fixed on the biochip then detected by a fluorescein-labelled secondary antibody. Fluorescence microscopy is utilized to visualize the fluorescence, and the intensity of fluorescence is evaluated by at least two experienced clinicians. A final diagnosis of NMOSD can be made based on the combination of anti-AQP4 IgG detection results, clinical manifestations, and neuroradiological findings. According to previous studies, CBA is more sensitive and specific than other anti-AQP4 IgG detection methods, and it can be applied to both clinical diagnosis and studies of NMOSD. The method has limitations; for example, an international scale to evaluate serum anti-AQP4 IgG titers is still lacking. Here, a detailed protocol for human serum anti-AQP4 IgG detection using CBA is described.
