Human Liver Spheroids from Peripheral Blood for Liver Disease Studies
PRÉPARATION DE L'INSTRUCTEUR
concepts
PROTOCOLE ETUDIANT
Ethical approval was obtained (ACA CELL Biotech GmbH/25b-5482.2-64-1) for performing these experiments and informed consent was signed by all donors before blood extraction in compliance with institutional guidelines.
1. Preparation of mononuclear cells (MNCs) from human peripheral blood (PB)
- Extract 30 mL of blood from healthy donors with the help of trained medical personnel according to the standard protocol.
- Isolate PBMNCs using density gradient media according to the protocol published by Becker-Kojić et al.13. Isolate, by pipetting, the interphase layer between plasma and density gradient media and use sterile phosphate buffer saline (PBS) to wash the isolated cells.
- Use a counting chamber and count the number of cells using standard methods.
2. Dedifferentiation of MNCs upon activation with human GPI-anchored glycoprotein
- Place 6 x 106 mononuclear cells in PBS/1% bovine serum albumin (BSA) in a polystyrene tube (15 mL) and incubate with the specific antibody for 30 min at 37 °C according to Becker-Kojić et al.13.
- Centrifuge the cells at 300 x g at room temperature and replace PBS/BSA with Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum (FBS).
- Grow the cells in 15 mL polystyrene tubes in a 5% CO2 incubator at 37 °C for 8-10 days as described in Becker-Kojić et al.13. On day 5 (D5), add 1-2 mL of Iscove's medium supplemented with 10% FBS to each 15 mL tube.
3. Sorting of newly generated dedifferentiated cells
- Centrifuge cultured cell suspension at room temperature for 10 min at 300 x g and aspirate with a sterile pipette the resulting supernatant according to Becker-Kojić et al.13.
- Resuspend pellet obtained by centrifugation in 90 µL of cold PBS buffer (PBS pH 7.2, 0.5 % BSA, and 2 mM EDTA) and add 10 µL of CD45 positive nano-sized magnetic beads and incubate on ice for 15 min.
- Wash the cell suspension with 2 mL of PBS buffer, centrifuge it at 300 x g for 10 min at room temperature and add 500 µL of PBS buffer to the cells and resuspend thoroughly.
- Pipette 500 µL of pre-cooled PBS buffer into the column to wash and place it in the magnetic field using a magnetic stand.
- Wash the cells placed on the column, twice with 500 µL of PBS buffer and collect flow-through containing CD45 negative cells in Iscove's medium supplemented with 10% FBS.
- Use a counting chamber to determine the number of reprogrammed cells.
4. Preparation of glass coverslips for the generation of human hepatocytes
- Separate glass coverslips (14 mm) and incubate them in nonionic detergent for 10 min. Wash glass coverslips in deionized water till no bubbles remain and incubate them in 1M HCl for 30 min (adapted from Marchenko et al.14).
- Wash glass coverslips with deionized water at least 3x and dry them overnight at room temperature. Autoclave dried glass coverslips in a suitable container.
5. Coating cell culture plates with biolaminin for 2D hepatic differentiation of BD-PSCs
- Place autoclaved glass coverslips with a sterile pair of tweezers in 4 well plates and turn UV lights on for 30 min to ensure sterile conditions.
- Thaw aliquots of biolaminin and add 120 µL of 5 µg/mL biolaminin to each glass cover slip. Leave the coated glass coverslips overnight at 4 °C.
- Remove the excess of biolaminin and add 200 µL of hepatocyte differentiation medium as described below.
6. Preparation of hepatocyte differentiation media
- Make 500 mL of hepatoblast knockout serum replacement (KSR)/dimethyl sulfoxide (DMSO) medium consisting of 76.4% knockout DMEM (KO-DMEM), 20% KSR, 0.5% L-glutamine, 1% non-essential amino acids (NEAA), 0.1% β-mercaptoethanol, 1% DMSO, and 1% penicillin-streptomycin (Pen/Strep).
- Prepare 500 mL of hepatocyte maturation medium containing 1% L-glutamine, 10 µM hydrocortisone 21-hemisuccinate sodium salt (HCC), and 1% Pen/Strep.
- Aliquot medium from the stock and add fresh hepatocyte growth factor (HGF) and oncostatin M (OSM) at final concentrations of 10 ng/mL and/or 20 ng/mL for each medium change.
NOTE: Oncostatin M is a cytokine belonging to the interleukin 6 group of cytokines, important for hematopoiesis as well as for liver development.
7. Culturing hepatic cells differentiated from BD-PSCs
- Put 3 x 105 BD-PSCS cells into each well of a 4-well plate coated with biolaminin.
- Place the 4-well plates in the incubator at 37 °C and 5% CO2. Culture the cells for 5 days in KSR/DMSO hepatoblast medium that supports endodermal differentiation and change the medium every second day.
- Switch to hepatocyte maturation medium on day 5 and culture the cells for an additional 7-10 days in the incubator at 37 °C and 5% CO2. Change the medium every 48 h.
8. 3D spheroid hepatic differentiation
- Count cells using a counting chamber.
- Centrifuge BD-dedifferentiated cell suspension at 300 x g for 10 min at room temperature. Remove supernatant and resuspend BD-dedifferentiated cells in KSR/DMSO medium at 2 x 106 cells/mL.
- Pass the cells through a 40 µm cell strainer to ensure a single-cell suspension and to remove any additional debris.
- Count cells using a counting chamber and prepare a sufficient volume for each cell seeding density in order to dispense the required volume per well. Prepare a gradient with top seeding of 1 x 106 cells to a low seeding density of 4000 cells.
- Dispense 100 µL of KSR/DMSO medium in 96 well-low attachment plates and add 100 µL of cell seeding dilution.
- Place low attachment plate in the incubator at 37 °C and 5% CO2 and culture them for 5 days.
- Change 50% of the medium from days 3-4 after seeding when the spheroids are sufficiently compact.
- On day 5, change the medium to hepatocyte maturation medium and culture the cells for an additional 7-10 days for further maturation. Change the medium every second day.
9. Immunofluorescence analysis of newly generated 2D liver cell cultures
- Culture the cells according to the differentiation method described above for 4, 8, 15, and 24 days and remove media.
- Incubate cells with pre-warmed fixative consisting of 4% paraformaldehyde in PBS for 10 min. Discard the fixative and wash the cells 2x with PBS for 5 min each. Immediately add 0.1% triton X-100 solution and permeabilize the cells for 5 min. Wash 2x with PBS.
- Add blocking solution made of PBS and 5% BSA and place on rocker plate for 1 h at room temperature.
- Dilute primary antibodies in dilution buffer 1% BSA/PBS as follows: albumin (ALB) 1:50, alpha-1 fetoprotein (AFP) 1:250, cytokeratin 18 (CK18) 1:50, hepatocyte nuclear factor 4 alpha (HNF4α) 1:1000 and transthyretin (TTR) 1:50. Use 50 µL of antibody dilution per well.
- Incubate the cells for 1 h at room temperature. Then discard the antibody solution, wash the cells for 5 min, and repeat the wash step 3x.
- Prepare the following secondary antibodies in dilution buffer: rabbit anti-chicken IgG (Texas red) 1:1000, goat anti-mouse IgG (488) 1:1000, and goat anti-rabbit (488) 1:500. Use 50 µL of antibody dilution per well and incubate the cells for 30 min at room temperature.
- Wash the cells 3x with PBS for 5 min each and mount the coverslips with mounting media containing DAPI for microscopic analysis.
10. Live staining of newly formed liver spheroids
- Carefully discard culture media while not touching the spheroids, add freshly made PBS with 0.1% triton X-100 solution, and incubate for 5 min to permeabilize the cells.
- Wash the spheroids with the medium by slowly pipetting for 5 min, repeat 2x.
- Incubate the spheroids with the primary antibodies ALB (1:50), AFP (1:250), CK18 (1:50), CYP2E1 (1:200), and CYP3A4 (1:200) prepared in PBS with 1% BSA for 1 h in 5% CO2 incubator at 37 °C. Use 50 µL of antibody dilution per well.
- Carefully remove the excess antibody solution and wash the spheroids with medium 3x.
- Prepare the corresponding secondary antibodies goat anti-mouse IgG (Cy3), goat anti-mouse IgG (488), and rabbit anti-chicken IgG (Texas red) at a dilution of 1:1000 and 1:500 for goat anti-rabbit (488) in PBS in 1% BSA. Use 50 µL of antibody dilution per well and incubate for 20 min in the incubator at 37 °C and 5% CO2.
- Carefully wash 3x with medium and leave the plate for 30 min in the incubator at 37 °C and 5% CO2 before performing fluorescence microscopy.
11. Examination of spheroids using a fluorescence microscope
- Switch on the fluorescence light source 10 min before use, turn on the computer and open the imaging software.
- Use an objective with 4x magnification, click on button 4x in the toolbar to have the correct scale bar selected, then place the 96-well plate on the stage center plate.
- Turn the LED light source on, use the brightfield filter, and position the plate to the well of interest using the x-y-axis stage adjustment knob.
- Change to camera light path, click the button Live in imaging software to visualize the image on the screen, and ensure the spheroid is centered using the x-y-axis knobs and focus using the coarse/fine focus knob.
NOTE: The shape of the spheroids remains constant after applying live staining. - Choose the Brightfield Observation method in the toolbar, put exposure settings to automatic, and click the button Snapshot in the camera control panel to take a picture. Then save the picture as .vsi file using the appropriate name in the folder of interest.
- Place ambient light shielding plate to turn off LED light, change to filter for B-excitation, choose 488 Observation method in the toolbar, open shutter, take a picture by clicking button Snapshot, close shutter, then save file as described above.
- Repeat this with a filter for G-excitation using the appropriate observation method (Texas red or CY3). Then reiterate the whole procedure for each well of interest.
- Return the plate to the 5% CO2 incubator at 37 °C and culture it as described above.
Human Liver Spheroids from Peripheral Blood for Liver Disease Studies
Learning Objectives
We successfully differentiated human BD-PSCs into endoderm/hepatic progenitor cells and hepatocytes by applying a two-step protocol. Morphological changes during the hepatic differentiation process are shown in Figure 1. BD-PSCs differentiate into hepatocytes going through three different stages. The first stage represents the differentiation into endodermal cells L4, the second, differentiation to hepatic progenitor cells (hepatoblast) L8, exhibiting a typical polygonal morphology, and the third, the maturation to hepatocytes L15-L24.
Immunofluorescence analysis was performed to confirm the hepatic differentiation of BD-PSCs as presented in Figure 2. Strong expression of endoderm/human liver progenitor marker, like alpha-fetoprotein (AFP), a major plasma protein in fetal serum whose concentration is very low in adult organisms and is therefore considered as a marker for hepatocytes' precursor15 and transthyretin (TTR), a major thyroid-hormone binding protein involved in transporting thyroxine from the bloodstream to the brain16 are found in the cells at the first stage of the hepatic differentiation process at L4 to L8. However, their expression decreases at L15, while the expression of albumin (ALB), the most abundant plasma protein produced mainly by the liver and utterly critical for hepatic differentiation, as well as hepatocyte nuclear factor 4 alpha (HNF-4α), a hepatocytes transcription factor that is involved in the expression of liver-specific genes17 appears firstly at L4, rises throughout the differentiation time L4-L15 reaching a strong and stable expression during the maturation time L15-L24.
Cytokeratin 18 (CK18) is a cytoskeletal protein, one of the major components of intermediate filament expressed in the liver18. The results show that, as expected, CK18 expression correlates with mature hepatocytes (L15-L24), and it is not expressed in hepatocyte progenitor cells.
The well-defined protocol for hepatocyte differentiation in 2D cultures enables the engineering of hepatic 3D cultures starting with BD-PSCs.
We demonstrate here that spontaneous aggregation of these cells in low attachment plates containing hepatocyte induction/maturation medium initiates spheroid formation. The growth track was followed by imaging cells at L2, L4, and L7. (Figure 3A) There is a consistent correlation between spheroid volume and the variable number of cells, as presented in Figure 3B.
The liver is an organ in which most of the drugs in the human body get metabolized. Cytochrome P450 is a superfamily of enzymes (monooxygenases) that are of pivotal importance in the processes of drug and cellular metabolism, detoxification of xenobiotics, and homeostasis. To assess the potential functional activity of BD-PSCs derived hepatic spheroids, we analyzed the expression of drug-metabolizing enzymes like CYP3A4 and CYP2E1, members of CYP3 and CYP2 families19.
Most of the drugs that are used today including codeine, cyclosporin A, erythromycin, acetaminophen, and diazepam as well as many steroids and carcinogens, are metabolized due to the activity of CY3A4 enzyme20. CYP2E1 is involved in the metabolism of endogenous substrates like ethylene glycol, benzene, carbon tetrachloride, and particularly the most important highly mutagenic compound like nitrosamine21.
The spheroids that are formed and differentiated according to the protocol at D14, live stained with antibodies to these two enzymes, reveal the potential hepatic functional activity of BD-PSCs derived spheroids (Figure 4).
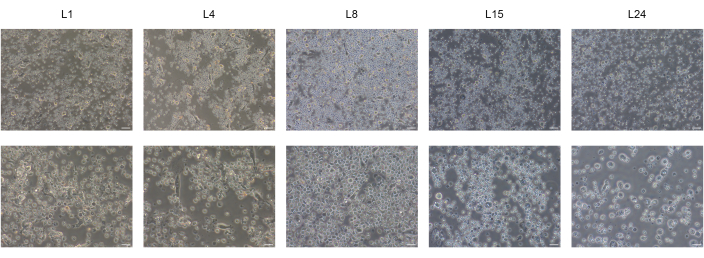
Figure 1: Differentiation of BD-PSCs to hepatic-like cells. Representative micrographs of morphological changes throughout hepatic differentiation of BD-PSCs showing endodermal L4, or polygonal shape L8 morphology finally reaching maturation state at L15 to L24. Scale bars: upper row 50 µm, lower row 20 µm. Please click here to view a larger version of this figure.
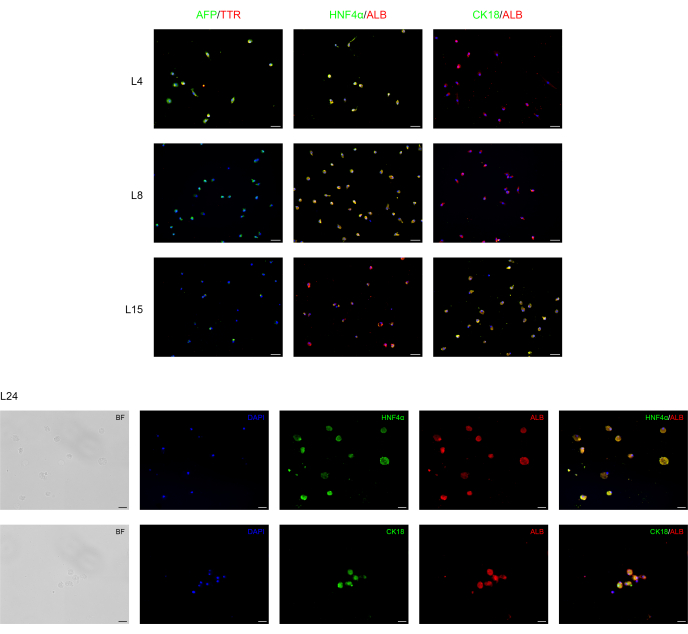
Figure 2: Immunofluorescence analysis of BD-PSCs re-differentiation towards hepatic cells. Endoderm/hepatocytes progenitor and hepatocytes specific markers are expressed during liver differentiation of BD-PSCs in 2D cultures. On days L4 to L8, micrographs show decreased expression of endoderm/hepatic progenitor AFP and TTR while their expression disappeared from L8-L24. Expression of hepatocytes ALB and HNFα markers arise at L4 and increase during maturation, whereas the expression of CK18 appeared first at L15, reaching the maximum at L24. Scale bars for graphs L4-L15: 50 µm and for L24: 20 µm. Control is presented in Supplementary Figure 1. Please click here to view a larger version of this figure.
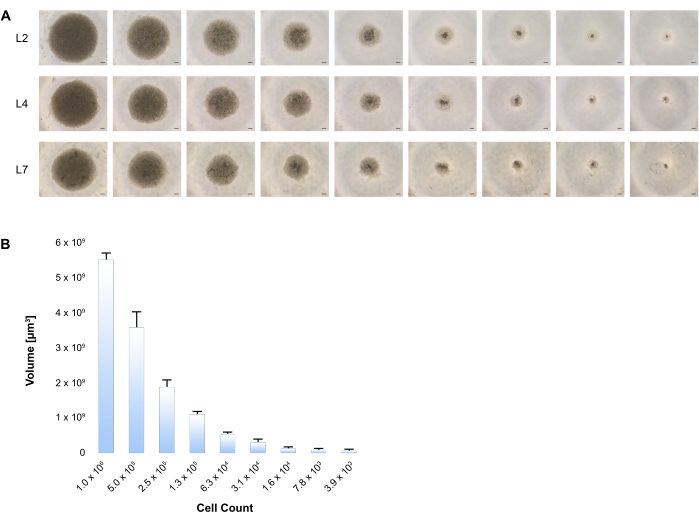
Figure 3: Formation of 3D spheroids upon hepatic differentiation of BD-PSCs. (A) Variable cell numbers of BD-PSCs starting with 1 x 106 to 4000 cells were seeded into low attachment plates, and differentiation was performed according to the two-stage procedure as stated in the Protocol. The generation of 3D human liver spheroids was imaged at different time points, shown are representative phase contrast images at each time during the culture time period. Scale bar: 200 µm. (B) Diameters of at least 4 hepatic spheroids for each size were measured at L4 using microscope imaging software and volumes were calculated. Error bars show standard deviation. Please click here to view a larger version of this figure.
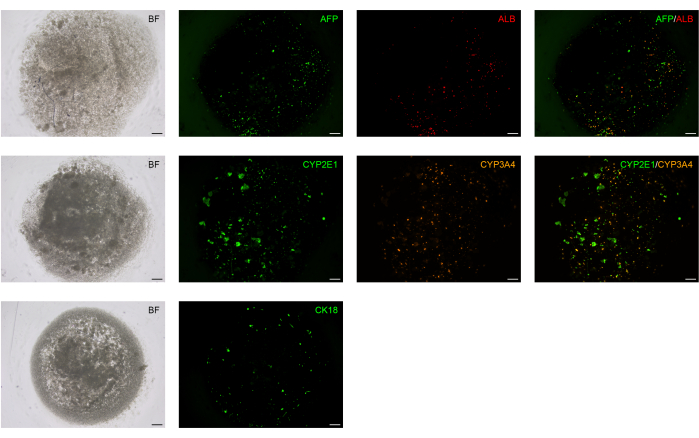
Figure 4: Hepatocyte functional markers are expressed in BD-PSCs-derived liver spheroids. BD-PSCs were differentiated into hepatocytes. Direct immunofluorescence analysis was performed on live cells at L14 using antibodies to ALB, AFP, CK18, and CYP2E1 and CYP3A4, members of the cytochrome P450 family. Scale bar: 200 µm. Please click here to view a larger version of this figure.
Supplementary Figure 1: Negative control for immunofluorescence analysis of BD-PSCs re-differentiation towards hepatic cells. Endoderm/hepatocytes progenitor and hepatocytes specific markers are expressed during liver differentiation of BD-PSCs in 2D cultures. Scale bar: 100 µm. Please click here to download this File.
List of Materials
| Albumin antibody | Sigma-Aldrich | SAB3500217 | produced in chicken |
| Albumin Fraction V | Carl Roth GmbH+Co. KG | T8444.4 | |
| Alpha-1 Fetoprotein | Proteintech Germany GmbH | 14550-1-AP | rabbit polyclonal IgG |
| Biolaminin 111 LN | BioLamina | LN111-02 | human recombinant |
| CD45 MicroBeads | Miltenyi | 130-045-801 | nano-sized magnetic beads |
| Cell Strainer | pluriSelect | 43-10040-40 | |
| CellSens | Olympus | imaging software | |
| Centrifuge tubes 50 mL | Greiner Bio-One | 210270 | |
| CEROplate 96 well | OLS OMNI Life Science | 2800-109-96 | |
| CKX53 | Olympus | ||
| Commercially available detergent | Procter & Gamble | nonionic detergent | |
| CYP2E1-specific antibody | Proteintech Germany GmbH | 19937-1-AP | rabbit polyclonal antibody IgG |
| CYP3A4 | Proteintech Germany GmbH | 67110-1-lg | mouse monoclonal antibody IgG1 |
| Cytokeratin 18 | DakoCytomation | M7010 | mouse monoclonal antibody IgG1 |
| DMSO | Sigma-Aldrich | D8418-50ML | |
| DPBS | Thermo Fisher Scientific | 14040091 | |
| FBS | Merck Millipore | S0115/1030B | Discontinued. Available under: TMS-013-B |
| Glass cover slips 14 mm | R. Langenbrinck | 01-0014/1 | |
| GlutaMax 100x Gibco | Thermo Fisher Scientific | 35050038 | L-glutamine |
| Glutaraldehyde 25% | Sigma-Aldrich | G588.2-50ML | |
| Goat anti-mouse IgG Cy3 | Antibodies online | ABIN1673767 | polyclonal |
| Goat anti-mouse IgG DyLight 488 | Antibodies online | ABIN1889284 | polyclonal |
| Goat anti-rabbit IgG Alexa Fluor 488 | Life Technologies | A-11008 | |
| HCl | Sigma-Aldrich | 30721-1LGL | |
| HepatoZYME-SFM | Thermo Fisher Scientific | 17705021 | hepatocyte maturation medium |
| HGF | Thermo Fisher Scientific | PHG0324 | human recombinant |
| HNF4α antibody | Sigma-Aldrich | ZRB1457-25UL | clone 4C19 ZooMAb Rbmono |
| Hydrocortisone 21-hemisuccinate (sodium salt) | Biomol | Cay18226-100 | |
| Knock out Serum Replacement – Multi Species Gibco | Fisher Scientific | A3181501 | KSR |
| KnockOut DMEM/F-12 | Thermo Fisher Scientific | 12660012 | Discontinued. Available under Catalog No. 10-828-010 |
| MACS Buffer | Miltenyi | 130-091-221 | |
| MACS MultiStand | Miltenyi | 130-042-303 | magnetic stand |
| MEM NEAA 100x Gibco | Thermo Fisher Scientific | 11140035 | |
| Mercaptoethanol | Thermo Fisher Scientific | 31350010 | 50mM |
| MiniMACS columns | Miltenyi | 130-042-201 | |
| Nunclon Multidishes | Sigma-Aldrich | D6789 | 4 well plates |
| Oncostatin M | Thermo Fisher Scientific | PHC5015 | human recombinant |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| PBS sterile | Carl Roth GmbH+Co. KG | 9143.2 | |
| Penicillin/Streptomycin | Biochrom GmbH | A2213 | 10000 U/ml |
| PS 15ml tubes sterile | Greiner Bio-One | 188171 | |
| Rabbit anti-chicken IgG Texas red | Antibodies online | ABIN637943 | |
| Roti Cell Iscoves MDM | Carl Roth GmbH+Co. KG | 9033.1 | |
| Roti Mount FluorCare DAPI | Carl Roth GmbH+Co. KG | HP20.1 | |
| Roti Sep 1077 human | Carl Roth GmbH+Co. KG | 0642.2 | |
| Transthyretin antibody | Sigma-Aldrich | SAB3500378 | produced in chicken |
| Triton X-100 | Thermo Fisher Scientific | HFH10 | 1% |
Lab Prep
Human liver cells can form a three-dimensional (3D) structure capable of growing in culture for some weeks, preserving their functional capacity. Due to their nature to cluster in the culture dishes with low or no adhesive characteristics, they form aggregates of multiple liver cells that are called human liver spheroids. The forming of 3D liver spheroids relies on the natural tendency of hepatic cells to aggregate in the absence of an adhesive substrate. These 3D structures possess better physiological responses than cells, which are closer to an in vivo environment. Using 3D hepatocyte cultures has numerous advantages when compared with classical two-dimensional (2D) cultures, including a more biologically relevant microenvironment, architectural morphology that reassembles natural organs as well as a better prediction regarding disease state and in vivo-like responses to drugs. Various sources can be used to generate spheroids, like primary liver tissue or immortalized cell lines. The 3D liver tissue can also be engineered by using human embryonic stem cells (hESCs) or induced pluripotent stem cells (hiPSCs) to derive hepatocytes. We have obtained human liver spheroids using blood-derived pluripotent stem cells (BD-PSCs) generated from unmanipulated peripheral blood by activation of human membrane-bound GPI-linked protein and differentiated to human hepatocytes. The BD-PSCs-derived human liver cells and human liver spheroids were analyzed by light microscopy and immunophenotyping using human hepatocyte markers.
Human liver cells can form a three-dimensional (3D) structure capable of growing in culture for some weeks, preserving their functional capacity. Due to their nature to cluster in the culture dishes with low or no adhesive characteristics, they form aggregates of multiple liver cells that are called human liver spheroids. The forming of 3D liver spheroids relies on the natural tendency of hepatic cells to aggregate in the absence of an adhesive substrate. These 3D structures possess better physiological responses than cells, which are closer to an in vivo environment. Using 3D hepatocyte cultures has numerous advantages when compared with classical two-dimensional (2D) cultures, including a more biologically relevant microenvironment, architectural morphology that reassembles natural organs as well as a better prediction regarding disease state and in vivo-like responses to drugs. Various sources can be used to generate spheroids, like primary liver tissue or immortalized cell lines. The 3D liver tissue can also be engineered by using human embryonic stem cells (hESCs) or induced pluripotent stem cells (hiPSCs) to derive hepatocytes. We have obtained human liver spheroids using blood-derived pluripotent stem cells (BD-PSCs) generated from unmanipulated peripheral blood by activation of human membrane-bound GPI-linked protein and differentiated to human hepatocytes. The BD-PSCs-derived human liver cells and human liver spheroids were analyzed by light microscopy and immunophenotyping using human hepatocyte markers.
Procédure
Human liver cells can form a three-dimensional (3D) structure capable of growing in culture for some weeks, preserving their functional capacity. Due to their nature to cluster in the culture dishes with low or no adhesive characteristics, they form aggregates of multiple liver cells that are called human liver spheroids. The forming of 3D liver spheroids relies on the natural tendency of hepatic cells to aggregate in the absence of an adhesive substrate. These 3D structures possess better physiological responses than cells, which are closer to an in vivo environment. Using 3D hepatocyte cultures has numerous advantages when compared with classical two-dimensional (2D) cultures, including a more biologically relevant microenvironment, architectural morphology that reassembles natural organs as well as a better prediction regarding disease state and in vivo-like responses to drugs. Various sources can be used to generate spheroids, like primary liver tissue or immortalized cell lines. The 3D liver tissue can also be engineered by using human embryonic stem cells (hESCs) or induced pluripotent stem cells (hiPSCs) to derive hepatocytes. We have obtained human liver spheroids using blood-derived pluripotent stem cells (BD-PSCs) generated from unmanipulated peripheral blood by activation of human membrane-bound GPI-linked protein and differentiated to human hepatocytes. The BD-PSCs-derived human liver cells and human liver spheroids were analyzed by light microscopy and immunophenotyping using human hepatocyte markers.
