Abstract
Source: Lopez-Ramirez M. A. et. al., Isolation and Culture of Adult Zebrafish Brain-derived Neurospheres. J. Vis. Exp. (2016).
This video describes the technique of microdissection of the brain from an adult zebrafish. This method helps in obtaining whole brain derived neuorospheres that can be studied further.
Protocol
1. Dissection of the Adult Zebrafish Brain
- Prepare a dissection bed by filling a 100 mm x 15 mm Petri dish with gel ice packs. Then place the lid on the petri dish and incubate at -20 °C until the gel freezes. On top of the lid place a square of clean filter paper and wrap both the filter paper and petri dish with plastic film.
- Clean and sterilize all the microdissection instruments by 70% ethanol or heat before each use. Place all sterilized dissection instruments near the dissecting microscope and, right before euthanasia, place the dissection bed under the microscope with optical fiber illumination.
- Collect 2 adult zebrafish for a whole brain neurosphere preparation; and 3 to 4 zebrafish to generate neurospheres from dissected brain regions.
- Euthanize adult zebrafish (8-12 months old) using a protocol approved by the Institutional Animal Care and Use Committee. Next, immerse the fish in 75% ethanol for 5-10 sec and quickly place in the dissection bed followed by decapitation at the level of the gills using a surgical blade.
- To euthanize animals, administer an overdose (300 mg/L) of tricaine methanesulfonate until the animal's heart-beat gradually slows down and circulation stops, then immerse in iced water.
- Turn the head dorsal side down, and using the scissors make a longitudinal cut from the cut side to the mouth. Using the forceps expose the base of the skull and remove all the adjacent tissue. Cut the lateral walls of the skull from the beginning of the spinal cord towards the tectum.
- Using the scissors, cut and remove the optic nerves and then remove both sides of the most lateral part of the skull at the level of the tectum. Turn the head ventral side up. Using forceps, peel off the rest of the most apical part of the skull.
- Transfer the brain along with the remaining part of the skull into a new dish with the dissection medium(DMEM/F12 with Penicillin /Streptomycin). Clean the brain tissue in the dissection medium using the micro knife's plastic handle, keeping all brain structures intact.
- From this point, continue the protocol using the whole zebrafish brain.
- Alternatively adapt this protocol to specific brain regions to generate neurospheres from whole zebrafish brain or from the telencephalon, tectum/diencephalon or cerebellum dissected with a fresh scalpel. Use a neural specific fluorescent zebrafish neural transgenic line to dissect the brain region of interest according to Figure 1.
Representative Results
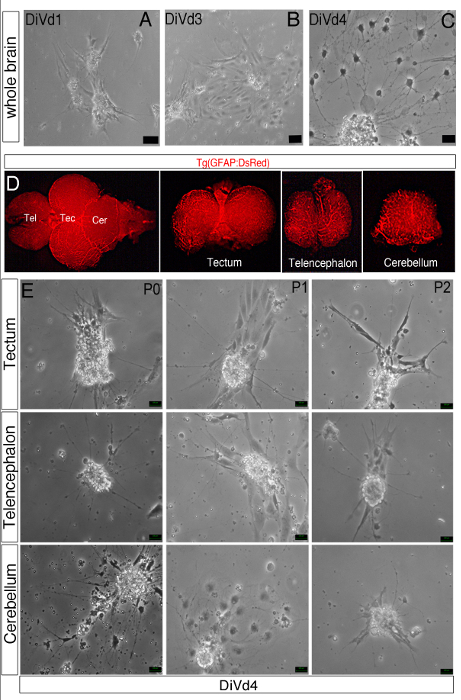
Figure 1: Differentiation of Zebrafish Brain-derived Neurospheres. (A–C, E) Phase contrast images of whole brain-derived neurospheres cultured in the Z-differentiation medium during 1 (A, DiVd1), 3 (B, DiVd3) and 4 (C and D, DiVd4) days. (E) Dorsal view of the whole brain of a 12 month old Tg(GFAP:DsRed) zebrafish. The telencephalon (Tel), tectum (Tec) and cerebellum (Cer) were dissected and collected as shown. (D) Images of DiVd4 neurospheres derived from the tectum, telencephalon and cerebellum at Passage 0 (P0), 1 (P1) and 2 (P2). Scale bar: 25 µm.
Materials
| DMEM/F12 1x | Life Technologies | 11330-032 | |
| Penicillin-streptomycin | Life Technologies | 15140-122 | |
| Tricaine MS-222 | Sigma | A5040 | stock solution of 4 mg/ml |

