Grafting Pancreatic Islets: A Method to Implant Pancreatic Islets into the Anterior Chamber of the Mouse Eye for In Vivo Imaging
Abstract
Source: Nilsson, J.et. al. A Longitudinal In Vivo Imaging and Quantification of Human Pancreatic Islet Grafting and Contributing Host Cells in the Anterior Eye Chamber. J. Vis. Exp. (2020).
This video describes the protocol for grafting human pancreatic islets into the anterior chamber of the mouse’s eye to monitor revascularization of human islet grafts in vivo. It also helps to understand the contribution of recipient versus donor cells in promoting the encapsulation and vascularization of the graft.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the jove veterinary review board
1. Preparation of transplantation equipment and surgery table
NOTE: All surgical tools should be autoclaved, and the surgery table and instruments disinfected with 70% alcohol.
- Connect a stereotaxic head holder to anesthesia via a nose mask and turn on the heating pad.
- Connect a gastight Hamilton syringe to polyethylene tubing and a blunt end eye cannula.
NOTE: It is recommended to fill all parts with PBS before assembly. Check for trapped air bubbles and remove if present. - Attach the Hamilton syringe tightly to the table (Figure 1a) or a movable base (Figure 1e) and attach the tubing to the stereo microscope, with cannula hanging down (i.e., waiting position).
NOTE: Use surgical tape, because it is easy to remove and reattach. - Prepare a 1 mL syringe connected to a 30 G needle filled with 0.1 mg/kg buprenorphine solution.
- Prepare a syringe with sterile PBS. Alternatively, use a pipette.
- Set aside a clean wake-up cage with a heating lamp.
2. Anesthesia and positioning of recipient mice for surgery
NOTE: All animals were bred and maintained in a pathogen-free environment at the animal facilities at Lund University.
- Anesthetize the mouse in a chamber filled with 40% O2/60% N2/3% isoflurane and transfer the anesthetized mouse to the head holder platform on a warm heating pad (Figure 1a). Check for the lack of pedal reflexes.
NOTE: Isoflurane anesthesia is the preferred method of anesthesia for fast recovery after surgery. The microscope room must be properly ventilated to use isoflurane. - Place the snout of the mouse into the anesthesia mask connected to 40% O2 /60% N2 0.9%–1.5% isoflurane anesthesia machine. Use the thumb and finger to lift the head up slightly and fasten it using the metallic pieces on the sides. Ensure that the earpieces fix the head directly below the ears. Inject 0.1 mg/kg buprenorphine solution subcutaneously on the back of the mouse.
NOTE: Buprenorphine is used as an analgesic. - Tilt the head so that the eye to be operated on is facing upwards and is close to the researcher.
- Gently retract the eyelids of the eye to be transplanted using blunt forceps, pop the eye out, and loosely fix with a pair of tweezers. Ensure that the tips of the tweezers are covered with a polythene tube attached to the head holder platform (Figure 1a, inset).
- Always keep both eyes wet by applying a droplet of sterile PBS onto the eye.
- Transfer the human islets from the sealed 1.5 mL tube (section 1) to a Petri dish with sterile PBS and make sure that the islets are close to each other to minimize the amount of cell culture media transferred (Figure 1c).
- Pick up ~20–30 islets in the eye cannula connected via polythene tubing to the Hamilton syringe.
NOTE: Take up as little liquid as possible with the islets. - Hang the tubing upside down and attach to the stereo microscope (Figure 1d). Tape the tubing carefully to let the islets sink to the end of the tube toward the cannula.
3. Transplantation procedure
NOTE: This method has been previously described for the transplantation of mouse islets. A slightly modified procedure is presented here.
- Pinch the pads on the hind legs to make sure that the mouse is asleep.
- Tighten the forceps restraining the eye without disrupting the blood flow and apply a droplet of sterile PBS onto the eye.
- Using a 25 G needle as a scalpel, bevel upwards, carefully penetrate only half of the tip in the cornea and make a single lateral incision. Make the hole in an upward angle; the hole will seal more easily after the transplant (Figure 1f).
- Carefully lift up the cornea with the cannula preloaded with islets and slowly apply islets in the eye. Avoid insertion of the cannula into the anterior chamber to prevent damage of the iris, but rather push carefully against the corneal opening (Figure 1g). Slowly retract the cannula from the ACE.
NOTE: Aim for an injection volume of 3–8 µL. If the volume is too large, it will expose the eye to unnecessarily high intraocular pressure and may result in reflux of the injected islets out of the anterior chamber. - When facing difficulties with insertion of the islets due to increased pressure in the eye chamber, enlarge the incision site by reinforcing the lateral incision site and reapply islets.
NOTE: Occasionally, introduced air bubbles can be used as space holders. - Apply eye gel to the eye, loosen the eye-restraining forceps and leave the mouse on isoflurane in the same position for 8–10 min to let the islets set.
- Remove the forceps holding the eyelid and put the eyelid back to its normal position.
- Remove the mouse from the head holder and transfer it to a wake up cage.
- When the mouse is awake and moving, transfer it back to the original cage and keep in the animal housing until scanning (at least 5 days are recommended).
Representative Results
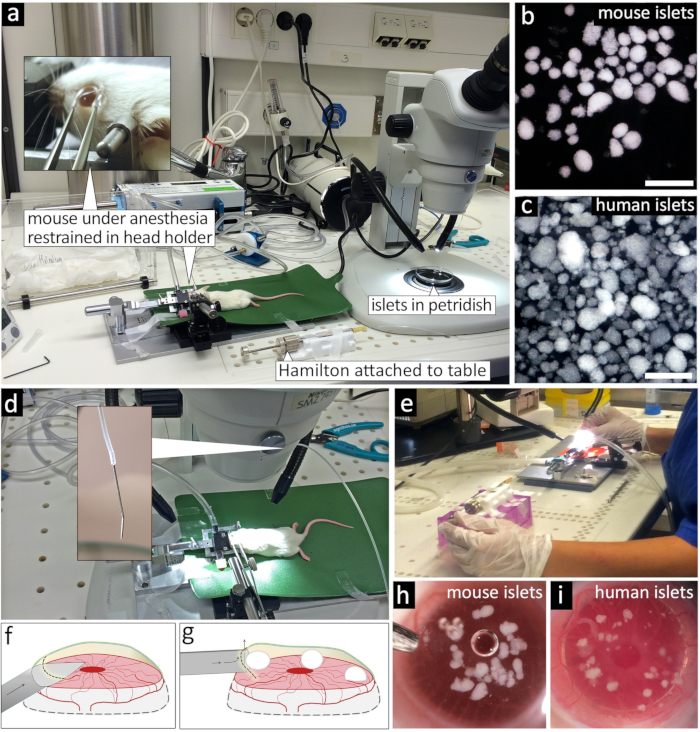
Figure 1: Transplantation of pancreatic islets into the anterior chamber of the eye.
(a) Transplantation setup showing anesthetized mouse fixed in stereotaxic head holder and the exposed eye (inset) next to the prepared Hamilton syringe fixed to the table and the stereomicroscope ready to pick mouse islets (b) or human islets (c). (d) Waiting position of the eye cannula loaded with islets. (e) Transplantation in process, and (f) schematic drawing of a single lateral incision used to carefully lift up the cornea with the tip of the eye cannula and dispense islets into the anterior chamber (g). Image of the eye immediately after injection of mouse islets (h) or human islets (i). Scale bar = 500 µm. Please click here to view a larger version of this figure.
Divulgazioni
The authors have nothing to disclose.
Materials
| Clean Bench coat | |||
| Heating pad | Set to 37 °C | ||
| Ivis Lumina ll Bioluminescent imager | Caliper | Alternative bioluminescent imaging systems include In vivo F PRO (Carestream) and Photon Imager (Biospace Lab) | |
| Dissecting scissors | |||
| Iris forceps (serrated) | |||
| Needle holder | |||
| 27 G 0.3 ml insulin syringe | Terumo | T35525M2913 |

