Fluid Aspiration from Mouse Glomeruli: A Method to Aspirate Glomerular Fluid from Mouse Using Two-Photon Mediated Micropuncture
Abstract
Source: Matsushita, K. et al. Micropuncture of Bowman's Space in Mice Facilitated by 2 Photon Microscopy. J. Vis. Exp. (2018)
This video showcases fluid aspiration from mouse glomeruli using the two-photon mediated micropuncture technique. The technique provides access to the living mouse’s glomeruli and thus helps gain insights into renal physiology.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Lateral Pipette Access to The Kidney Below a Fluid Imaging Column Via a Novel Surgical Procedure
NOTE: The assembly of the imaging support system and surgical prep is shown in Figure 1. The procedure described is performed on C57BL/6 mice weighing 20–25 g.
- Weigh the mouse.
- Induce anesthesia using 4% isoflurane and maintain with 1.5–2.5% isoflurane in air/oxygen mixture. Confirm that the mouse is anesthetized by absence of response to painful stimulus and reduced respiratory rate.
- Lubricate the eyes and position the animal lateral on a baseplate. Immobilize 4 extremities using tape.
- Inject normal saline, 200 µL, subcutaneously and place a rectal temperature probe. Control temperature using a heating lamp during surgery and a heating pad during imaging.
- Remove all hair on the left side of the mouse using a depilatory cream.
- Locate the spleen, which is visible under the skin, and locate the left kidney on the dorsal and caudal side of spleen.
- Make a 0.5 cm incision on the skin and smaller incision on the peritoneum, just enough for the kidney to push through easily.
- Extrude the kidney with gentle pressure. Place kidney stabilizer form made with polysiloxane around the kidney and fix with cyanoacrylate adhesive. Line the kidney up with the spacer such that the lateral-most surface of the kidney extends beyond the stabilizer by about 1 mm.
- Fix a head plate to the stabilizer form with glue and mount the head plate to mounting bars on the base plate.
- Fill the well in the polysiloxane support surrounding the kidney with 1% agarose solution and place the 10 mm coverslip on top and hold until agarose is firm. Seal coverslip to the head plate with glue and create a ring around the coverslip with dental cement.
- Inject FITC-dextran (2,000,000 Da, 5% solution, 100–150 µL) retro-orbitally and move the mouse and fixation plate to the 2-photon microscope stage quickly, maintaining anesthesia and ensuring adequate waste gas scavenging on the microscope stage.
2. Selection of a Suitable Glomerulus and Pipette Access to Bowman's Space
- Definitions:
- Define X as left-right on the screen and left-right facing the microscope
- Read SX (stage X) from the stage controller
- Define PX as pipette X, on the pipette dial controller
- Define Y as up-down on the screen and forward toward the microscope and back toward the 2-photon setup
- Define Z as up toward the ceiling, down toward the floor, and measured on the stage with the objective Z position.
- Define S(O)Z as the stage (really the objective) height.
- Find the surface of the kidney, identifiable using green fluorescent protein (GFP) filter settings in the ocular. Due to the injection of FITC-Dextran, the vasculature will be bright green.
- Identify a suitable glomerulus. After identifying the surface of the kidney using the ocular, switch to 2-photon (non-scanning mode) and explore the imaging window. Favorable characteristics for micropuncture are the following: vertical distance below the coverslip >30 µm (to prevent collision between the pipette and coverslip during access) and lateral distance from the lateral kidney capsule to the glomerulus <400 µm (beyond this distance the deviation of the pipette may increase likelihood of a miss).
- Record lateral and vertical distance to the puncture point, a point on the renal capsule directly to the pipette-side of the glomerulus.
- Raise the objective focal point into the water column, keeping the x and y stage coordinates unchanged, just about a centimeter.
- Drive the pipette tip into the water column and turn on 4',6-diamidino-2-phenylindol (DAPI) excitation. Move the pipette in the x and y dimensions to the point of maximal fluorescence of the tip, this will be the center of the objective. Finding the pipette in the ocular is difficult without this precise prepositioning. Because quantum dots fluoresce at the same (in this case, red) wavelength regardless of excitation wavelength, the DAPI excitation produces red fluorescence, which is tightly focused on the tip, illustrated in Figure 2.
- Change the excitation setting to red fluorescent protein (RFP) and visualize the pipette in the ocular, then precisely center it in the ocular view.
- Switch to 2-photon and find the pipette under 2-photon, placing it precisely in the center of the image. This is the registration position.
- Save an image of the pipette.
- Register the stage and the micropipette controller coordinates.
NOTE: Use the supplemental .html file, which will execute calculations using JavaScript code, (advantageous on systems which do not have installed spreadsheet software) or a spreadsheet to calculate the offset between stage and pipette coordinates and to calculate the target coordinates for the pipette controller. - Remove the pipette from the water column in the x axis, keeping z and y the same.
- Move the pipette Z to the target glomerulus Z (i.e., move the pipette down in the Z direction below the coverslip)
- Move the pipette Y to the target glomerulus Y coordinate.
- Move the 2-photon live view to the target glomerulus Z and then to the edge of the kidney and note the SX.
- Calculate the kidney edge PX using the offset from the registration SX.
- Move the stage toward the pipette (increase the SX) such that the edge of the kidney is far to the left of the screen, but still visible.
- Advance the pipette quickly to about 100 µm less than the kidney edge PX calculated above.
- Locate the pipette tip, advancing the pipette slowly. Increase the red gain and watch the red pixel histogram (the pixel distribution shifts before the pipette is imaged in the window, due to the extreme brightness of quantum dots and off-target fluorescence).
- Advance to the kidney edge under live 2-photon imaging.
NOTE: Prior to entering the renal capsule, it is possible to redirect the pipette in the Y and Z dimensions. However, this may break the pipette tip. A more conservative measure, if the pipette is off target, is to withdraw in the X dimension up to 2 cm, redirect, and then return in the X dimension to the kidney edge. Once the pipette is within the tissue, movement in any axis other than X leads to pipette flexion which requires great experience to make use of, and frequently leads to breakage. - Drive the pipette in the X axis slowly to the glom target PX, keeping an eye on the SX. (It is helpful to occasionally go back to the glomerulus to see if it has shifted at all upon insertion of the micropipette).
- When you are in the correct location, document position with a z-stack.
NOTE: With the micropipette in place, drugs, proteins, or fluorescent tracers may be injected, fluid may be aspirated for later analysis, or pressure or charge relative to another electrode may be measured.
3. Aspiration of Fluid from Bowman's Space
- Set the micropump to inject 100 nL of perfluorodecalin over 2 min to ensure patency of the pipette and reduce confounding from pipette plugging during entry. Reimage to ensure pipette position.
- Wait 4–6 min for additional filtration.
- Set the micropump to aspirate up to 300 nL at a rate of up to 50 nL/min.
NOTE: Changes in glomerular morphology are not observed with this rate, suggesting it does not alter the rate of delivery of fluid to the space during aspiration. As there is no oil block as in conventional micropuncture, recovery of this volume, necessary for mass spectrometry, could include some of the injected perflourodecalin and possibly tubular fluid. For assays such as ion-sensitive electrode measurements fluorescence spectroscopy, polymerase chain reaction, and other sensitive endpoints, lower volumes may be used. If mass spectrometry is not the endpoint, mineral oil and standard micropuncture techniques can be used to measure the aspirated volume prior to storage. - Image once more.
- Withdraw the pipette and preserve the sample, adding TRIS buffer and storing at -80° prior to analysis.
- Euthanize the mouse using an overdose of isoflurane or other approved method.
NOTE: Filtrate enters the space by filtration from the glomerular capillaries. The single-nephron glomerular filtration rate (SNGFR) in mice is reported between 8–14 nL/min. Starling forces govern SNGFR, however, and negative hydrostatic pressure in Bowman's space therefore may increase SNGFR. Standard micropuncture methods use tubular blockade with oil and neutral pressure for tubular fluid sampling, however these compounds interfere with mass spectrometry (see below); therefore, in this technique the early proximal tubule remains patent. Further, at the time of aspiration, Bowman's space contains an unknown, but positive volume of filtrate. Therefore, fluid aspiration rate may exceed SNGFR.
NOTE: In the experiments described here, the goal was to obtain a larger than usual sample of glomerular filtrate for mass spectrometry analysis by nanoproteomic techniques. Since use of mass spectrometry precludes use of oil blocks with mineral oil or wax (complex mixtures of organic molecules which reduce signal: noise in mass spectrometry) perfluorodecalin is used to fill the micropipette and syringe. Perflourodecalin is not known to block tubular flow but is biologically inert and does not interfere with mass spectrometry.
Representative Results
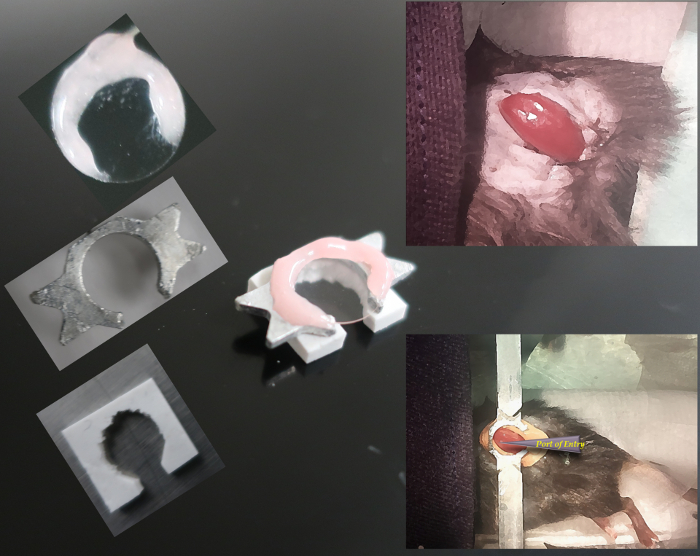
Figure 1: Partial extrusion of the kidney with custom support and immobilization for lateral access. On the left, the parts of the imaging column and kidney support are shown, with the complete assembly in center. On the right, the kidney preparation is shown before (above) and after (below) application of the support.
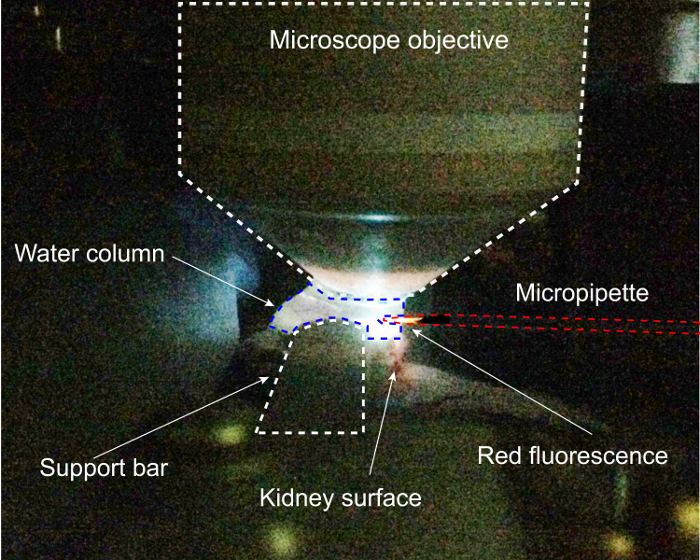
Figure 2: Completed kidney prep at pipette registration step of protocol. Here, DAPI excitation is being used to position the micropipette within the water column of the 2-photon microscope.
Divulgazioni
The authors have nothing to disclose.
Materials
| Upright 2 photon microscope | Zeiss | LSM 7MP | |
| 3 axis microscope stage controller | Sutter | MP-285 | |
| 3 axis headstage controller | Sutter | MP-225 | |
| Pipette holder | Molecular Devices | 1-HL-U | |
| Headstage | Molecular Devices | CV203BU | |
| FITC-dextran 2000 kDa MW | Sigma-Aldrich | 52471-1G | |
| Borosilicate glass capillary tubes | Sutter | B150-110-7.5 | |
| Micropipette puller | Sutter | P-97 | |
| Quantum dots, 605 nm | Thermofisher | Q21701MP | |
| Polysiloxane | Sugru | www.sugru.com, "original formula". Any color. | |
| PE-50 tubing | Instech Labs | BTPE-50 | |
| Microinjector | WPI | UMP-3 | |
| Microinjector controller | WPI | Micro4 | |
| Perfluorodecalin | Sigma-Aldrich | 306-94-5 | |
| Agarose | Sigma-Aldrich | 9012-36-6 | |
| Coverslip, 10 mm | Harvard Apparatus | 64-0718 | |
| Headplate | Custom | Common in neuroscience labs, many suppliers | |
| Head fixation device | Custom | Common in neuroscience labs, many suppliers | |
| 30G needle | Becton-Dickinson | 125393 | For retroorbital injection |
| Tuberculin syringe | Becton-Dickinson | 309626 | For retroorbital injection |

