Astrocyte Isolation: A Method to Obtain Pure Preparation of Mouse Cortical Astrocytes
Abstract
Source: Schildge, S. et al. Isolation and Culture of Mouse Cortical Astrocytes. J. Vis. Exp. (2013)
In this video, we describe the method to obtain a pure preparation of astrocytes from newly born mice.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Isolation and Plating of Mixed Cortical Cells
Mixed cortical cell isolation for astrocyte cultures can be performed using P1 to P4 mouse pups. In order to achieve proper astrocyte density it is necessary to use 4 mouse pup cortices per T75 tissue culture flask. Therefore, volumes in the following protocol are calculated for a cell preparation using 4 mouse pups.
- Before starting the dissection procedure, prewarm 30 mL of astrocyte culture media (DMEM, high glucose + 10% heat-inactivated fetal bovine serum + 1% Penicillin/Streptomycin; see table) to 37 °C. Coat one T75 flask with 20 mL of poly-D-lysine (PDL) at a concentration of 50 μg/mL in cell culture grade water for 1 hr at 37 °C in the CO2 incubator.
- For the dissection procedure, prepare all necessary reagents and materials. You will need surgical scissors, smooth fine forceps, flat tip forceps, paper towels, a waste bag, 70% ethanol, and 2 dissecting dishes (3.5 cm diameter) on ice filled with 2 mL HBSS each.
- Gently hold and spray the head and neck of the mouse pup with 70% ethanol. The animal is sacrificed by decapitation using scissors.
- Perform a midline incision, posterior to anterior, along the scalp to reveal the skull.
- Cut the cranium carefully from the neck to the nose. Two additional cuts are performed to allow further access to the brain: The first cut is made anterior of the olfactory bulbs, the other one inferior of the cerebellum to disconnect the cranium from the skull base.
- Using the flat tip forceps, the cranial flaps are gently flipped to the side and the brain is taken out and placed into the first dissecting dish filled with HBSS. Place the dish back on ice and continue harvesting all 4 brains.
- The remainder of the dissection procedure is performed under a stereomicroscope. First, the olfactory bulbs and the cerebellum are removed using the fine dissecting forceps (Figure 1B).
- In order to retrieve the cortices, grab the posterior end of the brain with the fine forceps, perform a midline incision between the hemispheres, insert a second set of forceps to the created grove and peel away the plate-like structure of the cortex from the brain.
- Carefully dissect the meninges from the cortex hemispheres by pulling with the fine forceps (Figure 1D'). This step avoids contamination of the final astrocyte culture by meningeal cells and fibroblasts. Transfer the prepared cortex hemispheres into the second dish filled with HBSS and return it onto ice. Continue accordingly with all 4 cortices.
- Finally, cut each cortex hemisphere into small pieces using sharp blades (approximately 4 to 8 times).
- Under sterile conditions, transfer cortex pieces into one 50 mL Falcon tube and add HBSS to a total volume of 22.5 mL.
- Add 2.5 mL of 2.5 % trypsin, mix and incubate the tissue in the water bath at 37 °C for 30 min. Mix by occasional shaking every 10 min.
- Centrifuge for 5 min at 300 x g to pellet cortex tissue pieces.
- Carefully remove supernatant by decantation. In order to avoid losing the tissue pellet you may mechanically retain it using a pipette. Dissociate the tissue into a single cell suspension by adding 10 mL astrocyte plating medium and vigorous pipetting using a 10 mL plastic pipette until tissue pieces are dissociated into single cells (20 to 30 times). Adjust volume to 20 mL using astrocyte plating medium. You can proof the dissociation of the cortex tissue into single cells by counting using a hematocytometer. One preparation of 4 mouse pup cortices should yield 10–15 x106 dissociated single cells.
- Aspirate PDL from the T75 culture flask, plate the dissociated single cell suspension and incubate at 37 °C in the CO2 incubator.
2. Obtaining an Enriched Astrocyte Culture
- Change the medium 2 days after plating of the mixed cortical cells and all 3 days thereafter.
- After 7 to 8 days, when astrocytes are confluent and overlaying microglia sit exposed on the astrocyte layer or are already detached from the astrocyte layer (Figure 2), shake the T75 flask at 180 rpm for 30 min on an orbital shaker to remove microglia. Discard the supernatant containing microglia or if you wish to culture and examine microglia, spin it down and plate for culture.
- Add 20 mL fresh astrocyte culture medium and continue by shaking the flask at 240 rpm for 6 hr to remove oligodendrocyte precursor cells (OPC). Since some OPCs will not completely detach from the astrocyte layer, continue to shake vigorously by hand for 1 min in order to prevent OPC contamination. Discard the supernatant or spin it down and plate, if you wish to culture OPCs.
- Rinse the remaining confluent astrocyte layer twice with PBS, aspirate the PBS, add 5 mL trypsin-EDTA and incubate in the CO2 incubator at 37 °C. Check detachment of astrocytes every 5 min and enforce detachment of astrocytes by hitting the flask against the palm of your hand (2–3 times).
- After astrocytes are detached from the culture flask, add 5 mL of astrocyte culture medium, spin cells at 180 x g for 5 min, aspirate supernatant and add 40 mL fresh astrocyte plating medium. One T75 tissue culture flask should yield around 1×106 cells after the first cell split. Plate cells in two T75 culture flasks and incubate at 37 °C in the CO2 incubator. Change the medium every 2 to 3 days.
- 12–14 days after the first split astrocytes are plated in the appropriate cell concentration 24–48 hr before performing the experiment. One T75 tissue culture flask should yield around 1.5–2 x106 cells after the second cell split.
Representative Results
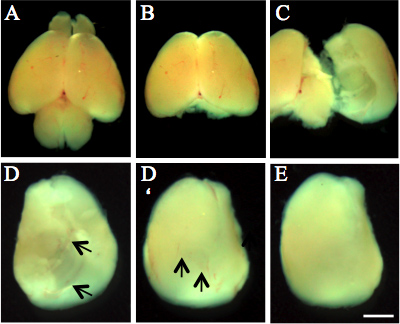
Figure 1. Dissection of postnatal (P3) mouse cortex. A) Whole brain. B) Brain after removal of olfactory bulbs and cerebellum. C) Isolation of cortices by peeling off the plate-like structure of the cortex from the brain. D, D') Cortex from ventral and dorsal site with meninges (black arrows indicate meningeal arteries). E) Cortex without meninges. Scale bar, 1.5 mm.
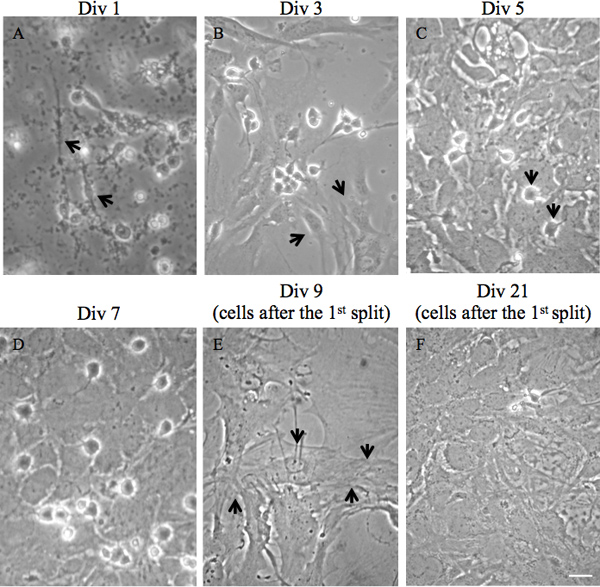
Figure 2. Morphological overview of isolated mixed cortical cells and pure astrocyte culture at different timepoints after isolation. A) 1 day after plating of mixed cortical cells. First astrocytes are attached to the bottom of the flask (black arrows) and dying neurons are in the supernatant. B) 3 days after plating of mixed cortical cells. Astrocyte layer is forming (black arrows). Neurons are almost absent. C) 5 days after plating of mixed cortical cells. First microglia and OPCs on top of a astrocyte layer (black arrows). D) 7 days after plating of mixed cortical cells. Astrocyte layer is completely confluent. E) After removing microglia and OPCs by vigorous shaking and 2 days after splitting, attached cells show astrocyte morphology with low density (arrows indicate one cell). F) Astrocyte layer shows high density 2 weeks after the first split. Scale bar, 10 μm.
Divulgazioni
The authors have nothing to disclose.
Materials
| Astrocyte culture media | |||
| DMEM high glucose | Life Technologies | 31966-021 | |
| FBS heat-inactivated | Life Technologies | 10082-147 | Final Concentration: 10% |
| Penicillin-Streptomycin | Life Technologies | 15140-122 | Final Concentration: 1% |
| Solution for brain tissue digestion | |||
| HBSS | Life Technologies | 14170-088 | |
| 2.5% Trypsin | Life Technologies | 15090-046 | Final Concentration: 0.25% |
| Altro | |||
| 70% (vol/vol) ethanol | Roth | 9065.2 | |
| Poly-D-Lysine | Millipor A-003 | A-003-E | |
| Water | PAA | S15-012 | Cell culture grade |
| PBS | PAA | H15-002 | Cell culture grade |
| 0.05% Trypsin-EDTA | Life Technologies | 25300-062 | |
| 0.45 µm Sterile filter | Sartorius | 16555 | |
| 3.5 cm petri dish | BD Falcon | 353001 | |
| 15 ml Falcon tube | BD Falcon | 352096 | |
| 50 ml Falcon tube | BD Falcon | 352070 | |
| 75 cm2 Tissue culture flask | BD Falcon | 353136 | |
| Forceps fine | Dumont | 2-1032; 2-1033 | #3c; #5 |
| Forceps flat tip | KLS Martin | 12-120-11 | |
| 13 cm surgical scissors | Aesculap | BC-140-R | |
| Stereomicroscope | Leica | MZ7.5 | |
| Stereomicroscope + Camera | Leica | MZ16F; DFC320 | |
| Microscope + Camera | Zeiss; Canon | Primo Vert; PowerShot A650 IS | |
| Centrifuge | Eppendorf | 5805000.017 | Centrifuge5804R |
| Orbital Shaker | Thermo Scientific | SHKE 4450-1CE | MaxQ 4450 |
| Water bath | Julabo | SW20; 37 °C |

