Isolation of Cerebral Capillaries from Human Brain: An Optimized Method to Extract Cerebral Capillaries from Human Brain Tissue
Abstract
Source: Hartz, A. M. S. et al. Isolation of Cerebral Capillaries from Fresh Human Brain Tissue. J. Vis. Exp. (2018)
This video presents the isolation of cerebral capillaries from a fresh human brain tissue. The isolated capillaries can then be used to generate an ex vivo blood brain barrier model.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
1. Brain Sample Preparation
NOTE: Figure 1A shows the workflow chart of the entire isolation procedure described below. Human brain tissue can stem from any part of the cortex and can be used fresh or frozen. Frozen brain tissue can be thawed at room temperature (no buffer; ~30 min for 10 g). To achieve comparable results, the brain tissue should be obtained from the same brain region for each experiment. This protocol is optimized for fresh (Post-Mortem Interval or PMI <4 h) human cerebral cortex that has not been frozen.
- Preparation of human brain tissue: Document the weight of the brain tissue. All numbers in the following protocol are appropriate for 10 g of fresh human brain tissue. Place the brain tissue in a 100 mm Petri dish. Carefully remove all the meninges with forceps. Use a scalpel to cut off the white matter.
- Mincing of the human brain tissue: Carefully cut up the brain tissue and mince it with a scalpel. Mince for about 5 min (2–3 mm pieces). Transfer the brain tissue to the Potter-Elvehjem tissue grinder. Add 30 mL of isolation buffer.
NOTE: The minced tissue pieces are difficult to see since the brain tissue turns into mush through the mincing process.
2. Homogenization
- Potter-Elvehjem tissue grinder (clearance: 150–230 µm): Homogenize each sample with 100 strokes at a homogenizer speed of 50 rpm. Document the time every 25 strokes and the total time needed for 100 strokes. See Table 1 for a proposed homogenization protocol; the total time for homogenizing 10 g of human frontal cortex is about 22 min. Do not stir in air to prevent bubbles.
- Dounce homogenizer (clearance: 80–130 µm): Transfer the homogenate to a Dounce homogenizer on ice. Homogenize the suspension with 20 strokes (~6 s/stroke, total of ~2 min). Avoid bubbles.
3. Centrifugation
- Distribute the brain homogenate equally into four 50 mL centrifugation tubes and document the total volume of the homogenate. Distribute 50 mL of density gradient buffer into the centrifugation tubes (12.5 mL per tube). Use 10 mL of isolation buffer to rinse the pestle and homogenizer and distribute into the four centrifugation tubes (~2.5 mL per tube).
- Tightly close the centrifuge tubes with caps. Mix the homogenate, density gradient medium, and buffer by vigorously shaking the tubes. Centrifuge at 5,800 x g for 15 min at 4 °C (fixed angle rotor); select a medium deceleration speed to keep the pellet attached to the tube. Discard the supernatant and resuspend each pellet in 2 mL of 1% BSA.
4. Filtration
NOTE: To separate the capillaries from red blood cells and other cell debris, several filtration steps are necessary.
- 300 µm mesh: After resuspending the pellet, filter the suspension through the 300 µm mesh. Capillaries are filtered through the mesh, whereas larger vessels and larger brain debris remain on the mesh. Carefully wash the mesh with up to 50 mL of 1% BSA. Discard the mesh.
NOTE: This filtration step clears the capillary suspension from any larger vessels or chunks of brain debris. - 30 µM cell strain filter
NOTE: This filtration step separates capillaries from red blood cells and other brain debris.- Distribute the capillary filtrate from step 4.1 over the five 30 µm cell strain filters (about 10 mL of capillary filtrate per cell strain filter). Capillaries are held back by this filter, whereas red blood cells, other single cells, and small brain debris pass through the filter and are collected in the filtrate.
- Wash each filter with 25 mL of 1% BSA. Afterwards, pour all filtrates over the sixth filter to increase the yield. Wash each filter with 50 mL of 1% BSA; keep the cell strain filters with containing the capillaries and discard the filtrate.
5. Capillary Collection
- Turn the filters upside down and wash the capillaries with 50 mL of 1% BSA for each filter into 50 mL tubes. Gently apply pressure with the pipet tip of a 5 mL pipettor and move it across the filter to wash off the brain capillaries.
- Make sure to wash off all brain capillaries, especially from the rim of the filter. Avoid bubbles since this makes the filtration process more difficult and increases the chance of capillary loss.
6. Washing
- After collecting the capillaries, centrifuge all samples at 1,500 x g for 3 min at 4 °C (swinging bucket rotor). Remove the supernatant and re-suspend the pellet in approximately 3 mL of isolation buffer. Combine all resuspended pellets from one sample in a 15 mL conical tube and fill it with isolation buffer. Centrifuge again at 1,500 x g for 3 min at 4 °C and wash two more times.
- Document the capillary purity with a microscope (100X magnification) and camera (Figure 1B).
NOTE: The brain capillary yield from 10 g of human brain tissue is usually about 100 mg. The isolated brain capillaries can now be used for experiments, processed (e.g., lysate, membrane isolation), or be flash-frozen and stored at -80 °C in cryotubes for a minimum of 6–12 months (avoid multiple freeze-thaw cycles).
| Strokes | Time |
| 1–25 | 7–7.5 |
| 26–50 | 5–5.5 |
| 51–75 | 5–5.5 |
| 76–100 | 5–5.5 |
| Total Time: | 22–24 min |
Table 1: Homogenization protocol. The homogenization protocol for the Potter-Elvehjem tissue grinder to homogenize 10 g of human frontal cortex at a homogenization speed of 50 rpm. Note that the first several strokes require additional time to homogenize the minced tissue. After this initial homogenization, each stroke is 12 s in duration (6 s for downward movement, 6 s for upward movement). Thus, after the initial homogenization, 5 strokes can be accomplished in 1 min, or 25 strokes in 5 min.
Representative Results
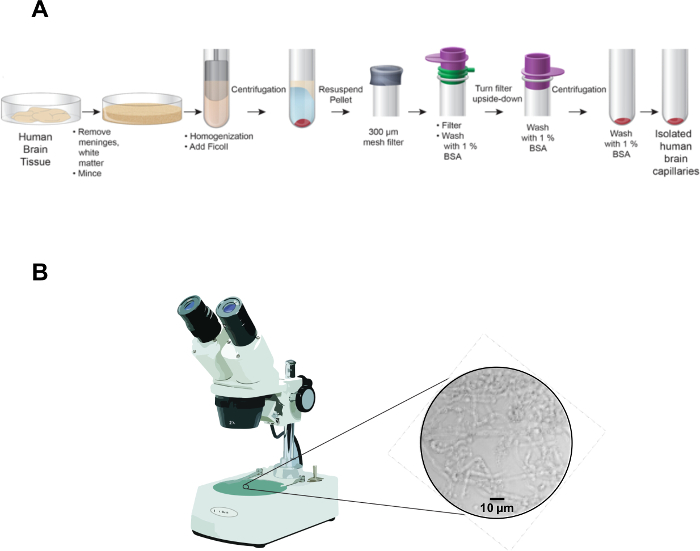
Figure 1: Flowchart for capillary isolation. (A) The pictogram illustrates major steps of the procedure to isolate brain capillaries from fresh human tissue. (B) The picture shows isolated human brain capillaries under a light microscope directly after isolation (100X magnification).
Divulgazioni
The authors have nothing to disclose.
Materials
| Personal Protective Equipment (PPE) | |||
| Diamond Grip Plus Latex Gloves, Microflex Medium | VWR, Radnor, PA, USA | 32916-636 | PPE |
| Disposable Protective Labcoats | VWR, Radnor, PA, USA | 470146-214 | PPE; due to the nature of the human source material, the use of a disposable lab coat is recommended |
| Face Shield, disposable | Thermo Fisher Scientific, Pittsburgh, PA, USA | 19460102 | PPE; due to the nature of the human source material, the use of a disposable face shield is recommended |
| Safety Materials | |||
| Clavies High-Temperature Autoclave Bags 8 x 12 | Thermo Fisher Scientific, Pittsburgh, PA, USA | 01-815-6 | |
| Versi Dry Bench Paper 18" x 20" | Thermo Fisher Scientific, Pittsburgh, PA, USA | 14-206-32 | To cover working areas |
| VWR Sharps Container Systems | Thermo Fisher Scientific, Pittsburgh, PA, USA | 75800-272 | For used scalpels |
| Bleach 8.2% Clorox Germicidal 64 oz. | UK Supply Center, Lexington, KY, USA | 323775 | |
| Equipment | |||
| 4 °C Refrigerator | Thermo Fisher Scientific, Pittsburgh, PA, USA | 13-986-148 | |
| Accume BASIC AB15 pH Meter | Thermo Fisher Scientific, Pittsburgh, PA, USA | AB15 | |
| Heidolph RZR 2102 Control | Heidolph, Elk Grove Village, IL, USA | 501-21024-01-3 | |
| Sorvall LEGEND XTR Centrifuge | Thermo Fisher Scientific, Pittsburgh, PA, USA | 75004521 | |
| Leica L2 Dissecting Microscope | Leica Microsystems Inc, Buffalo Grove IL, USA | Used to remove meninges | |
| POLYTRON PT2500 Homogenizer | Kinematica AG, Luzern, Switzerland | 9158168 | |
| Scale P-403 | Denver Instrument, Bohemia, NY, USA | 191392 | |
| Standard mini Stir | Thermo Fisher Scientific, Pittsburgh, PA, USA | 1151050 | |
| Thermo-Flasks Liquid Nitrogen Dewar | Thermal Scientific, Mansfiled, TX, USA | 11-670-4C | Used to freeze the tissue |
| Voyager Pro Analytical Balance | OHAUS, Parsippany, NJ, USA | VP214CN | |
| ZEISS Axiovert Microcope | Carl Zeiss, Inc Thornwood, NY, USA | Used to check isolated capillaries | |
| Tools and Glassware | |||
| Finnpipette II Pipette 1-5 mL | Thermo Fisher Scientific, Pittsburgh, PA, USA | 21377823T1 | Wash capillaries off filter |
| Finnpipette II Pipette 100-1,000 µL | Thermo Fisher Scientific, Pittsburgh, PA, USA | 21377821T1 | Resuspend pellet in BSA |
| Pipet Boy | Integra, Hudson, NH, USA | 739658 | |
| 50 mL Falcon tubes 25/rack – 500/cs | VWR, Radnor, PA, USA | 21008-951 | |
| EISCO Scalpel Blades | Thermo Fisher Scientific, Pittsburgh, PA, USA | S95938C | To mince brain tissue |
| PARAFILM | VWR, Radnor, PA, USA | 52858-000 | To cover beaker and volumetric flask |
| Thermo Scientific Finntip Pipet Tips 5 mL | Thermo Fisher Scientific, Pittsburgh, PA, USA | 21-377-304 | To wash capillaries off filter |
| 60 mL syringe with Luer-Lok | Thermo Fisher Scientific, Pittsburgh, PA, USA | BD309653 | Used with connector ring to filter capillaries |
| Scalpel Handle #4 | Fine Science Tools, Foster City, CA, USA | 10060-13 | Used for mincing |
| Dumont Forceps #5 | Fine Science Tools, Foster City, CA, USA | 11251-10 | Used to remove meninges |
| Potter-Elvehjem Tissue Grinder | Thomas Scientific, Swedesboro, NJ, USA | 3.43E+28 | 50 mL volume, clearance: 150-230 μm |
| Dounce Homogenizer | VWR, Radnor PA USA | 62400-642 | 15 mL volume, clearance: 80-130 μm |
| Spectra/Mesh Woven Filters (300 µm) | Spectrum Laboratories, Rancho Dominguez, CA, USA | 146424 | Used to filter capillary suspension to remove any meninges that may be left |
| pluriStrainers (pore size: 30 µm) | pluriSelect Life Science, Leipzig, Germany | 43-50030-03 | |
| Connector Ring | pluriSelect Life Science, Leipzig, Germany | 41-50000-03 | Reuse multiple time |
| 1 L Volumetric Flask | For preparation of Isolation Buffer | ||
| 1 L Beaker | For preparation of 1% BSA | ||
| Stir Bar | For preparation of 1% BSA and Ficoll® | ||
| Schott Bottle (60 mL) | For preparation of Ficoll® | ||
| Ice Bucket | To keep everything cold | ||
| 100 mm Petri dish | For mincing of brain tissue | ||
| Tissue Culture Cell Scraper | VWR, Radnor, PA, USA | 89260-222 | To remove supernatant after centrifugation |
| Chemicals | |||
| BSA Fraction V, A-9647 | Sigma-Aldrich, St. Louis, MO, USA | A9647-500g | Prepare in DPBS with Ca2+ & Mg2+ the day before. Avoid bubbles during preparation. Store in the refrigerator. Slowly stir for 10 min before use. |
| DPBS with Ca2+ & Mg2+ | Hyclone | SH30264.FS | DPBS – part of the Isolation Buffer |
| Ficoll PM400 | Sigma-Aldrich, St. Louis, MO, USA | F4375 | Exact measurement is important here. Weigh out in bottle with stir bar. Shake vigurously after adding DPBS. Keep in the fridge O/N. It will be clear in the morning. Stir gently for 10-15 min before use. Keep on ice until use. |
| Glucose (D-(+) Dextrose) | Sigma-Aldrich, St. Louis, MO, USA | G7528 | Glucose (D-(+) Dextrose) Concentration: 5 mM |
| Sodium Hydroxide Standard Solution | Sigma-Aldrich, St. Louis, MO, USA | 71474 | To adjust pH of the DPBS |
| Sodium Pyruvate | Sigma-Aldrich, St. Louis, MO, USA | P2256 | Concentration: 1 mM |

