Catheter Based Intravenous Injection of Experimental Agents: A Technique for Delivering Experimental Agents Intravenously Through Jugular Vein in Mouse Model
Abstract
Source: Bornert, A. et al. In Vivo Two-photon Imaging of Megakaryocytes and Proplatelets in the Mouse Skull Bone Marrow. J. Vis. Exp. (2021)
In this video, we describe a method for implanting a catheter into the jugular vein of a mouse model through which experimental agents such as drugs and tracer dyes can be delivered intravenously in vivo. Such an arrangement when connected to other systems can also be used for monitoring hemodynamics and blood sampling.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of mice and insertion of a catheter in the jugular vein
NOTE: Here, male or female, 5-7-week-old mTmG reporter mice were used (B6.129(Cg) Gt(ROSA)26Sortm4(ACTBtdTomato,-EGFP)Luo10) crossed with Pf4-cre mice, allowing intense green fluorescence labeling in megakaryocytes and platelets. Before beginning the experiment, preheat the heating chamber of the microscope for a few hours.
- Transport the mouse to the imaging room and proceed to anesthesia.
- Anesthetize the mouse by intraperitoneal (i.p.) injection of a solution of ketamine (ketamine (100 μg/g) and xylazine (10 μg/g) (5 μL/g).
NOTE: Repeated administration of ketamine/ xylazine is used here and works well but requires the interruption of recording for regular reinjection. Alternatively, if an anesthesia machine is available, the induction of anesthesia may be performed with ketamine/xylazine and subsequently maintained under inhalation of gases (mixture of isoflurane, air, and oxygen). - Replace the animal in its cage until it reaches deep anesthesia. Place the mouse on a heated plate warmed at 37 °C for all subsequent manipulations.
NOTE: While the animal reaches deep anesthesia, switch on the microscope and all equipment and set up the different parameters needed to start the experiment once the surgery is done. Here, the procedure lasts only 3 h and is terminal. The mouse is euthanized after the experiment without waking up. Hence, ethanol disinfection for skin and instruments is sufficient. However, depending on the institutional guidelines, or if the procedure would last longer, more complete disinfection methods should be used such as three alternating scrubs with betadine/povidone and alcohol.
- Anesthetize the mouse by intraperitoneal (i.p.) injection of a solution of ketamine (ketamine (100 μg/g) and xylazine (10 μg/g) (5 μL/g).
2. Install a catheter in the jugular vein.
NOTE: It is important to avoid microbial contamination during the surgery as far as possible. Take care to carefully disinfect the skin with 70% ethanol (EtOH) before proceeding with surgery. Always use sterilized scissors and tweezers, and regularly rinse them in 70% EtOH. Work in a clean place and wear a face mask. A 22 G "over-the needle" catheter, consisting of a needle inside a plastic tube, is used for intravenous injection (see the Table of Materials).
- Place the mouse on its back and fix the anterior legs with surgical tape to stretch the throat (Figure 1A). Disinfect the throat with 70% ethanol.
NOTE: For convenience, the throat may be shaved before surgery. If the procedure lasts longer, careful shaving must be performed for proper aseptic technique. - Make a 0.7-1 cm incision over the right or left jugular vein using sterile scissors. Stretch the adjacent connective tissue to expose the external jugular vein and the top of the pectoral muscle (Figure 1A).
- Fill the catheter with warm, sterile physiological saline. Insert the catheter into the vein after penetrating through the pectoral muscle (Figure 1B).
NOTE: Take care to avoid air bubbles inside the catheter. It is important to insert the catheter through the muscle to prevent hemorrhage as the muscle will form a compression point. Even mice with hemostasis defects, such as Itgb3-/- mice, do not bleed upon catheter insertion with this approach. - Gently remove the needle, and if required, carefully move the catheter deeper into the vein. Connect the syringe and stabilize the catheter by adding a drop of surgical glue (Table of Materials).
NOTE: It is possible to insert a catheter inside the tail vein, although it requires some practice because the vein has a smaller caliber. This is especially difficult when using mice with dark hair, whose tail vein is hardly visible. Due to its larger caliber, positioning the catheter inside the jugular vein may allow larger volumes or repeated treatments to be injected more reliably. Please note that the syringe is filled at first time with warm saline, and do not forget to inject the dead volume before injecting the drug solution. - Carefully return the mouse to a prone position (Figure 1C). Apply gel (Table of Materials) on the eyes to prevent dryness.
Representative Results
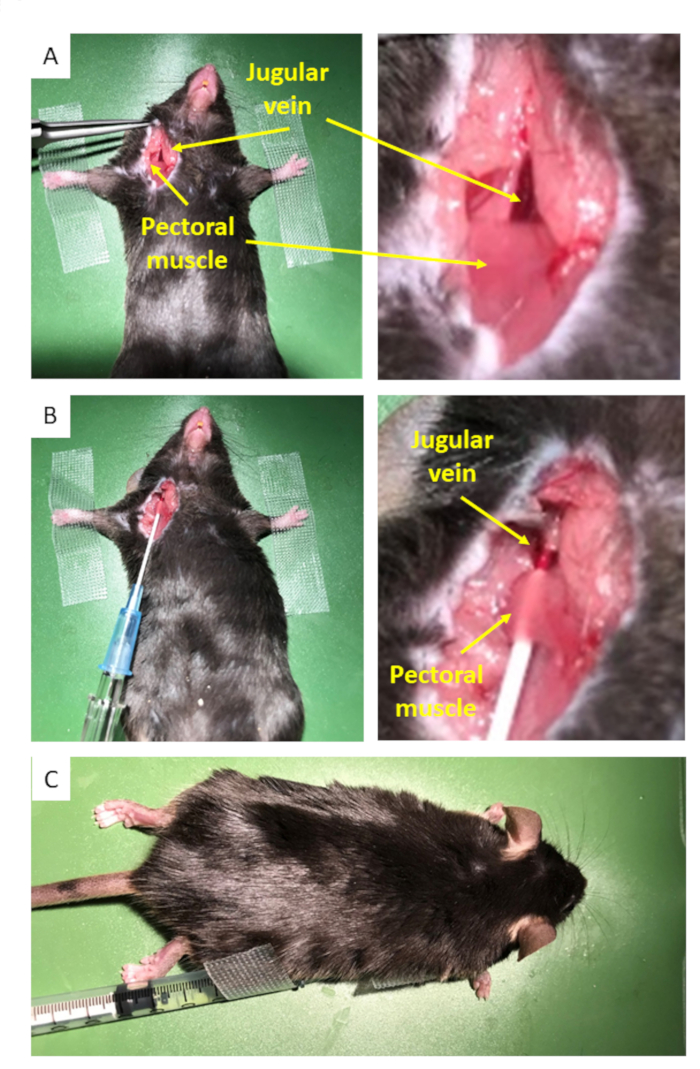
Figure 1: Insertion of the catheter. (A) An incision is made to expose the jugular vein and the top of the pectoral muscle. (B) The catheter filled with saline is inserted in the jugular vein by passing through the muscle, creating a compression point. (C) The mouse is carefully turned so that it lies ventral surface downward with the catheter well-positioned.
Divulgazioni
The authors have nothing to disclose.
Materials
| Histoacryl 5 x 0, 5 mL | Braun | 1050052 | Injectable solution of surgical glue |
| Imalgene/Ketamine 1000 fl/10 mL | Boehring | 3661103003199.00 | Eye protection |
| Rompun Xylazine 2% fl/25 mL | Bayer | 4007221032311.00 | |
| Surflo IV catheter – Blue 22 G | Terumo | SR-OX2225C1 |

