Isolating RPE and IPE Cells from Bovine Eye: A Procedure to Harvest and Culture Bovine Primary Pigment Epithelial Cells
Abstract
Source: Bascuas, T. et al., Isolation, Culture, and Genetic Engineering of Mammalian Primary Pigment Epithelial Cells for Non-Viral Gene Therapy. J. Vis. Exp. (2021).
This video demonstrates the procedure to isolate the retinal pigment epithelial, or RPE, cells and iris pigment epithelial, or IPE, cells from harvested bovine eyes. The isolated cells can be cultured to study their role in the treatment of ocular diseases.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Isolation of bovine PE cells
- Use curved scissors and Colibri forceps to enucleate the eyes after euthanizing the animal. Clean the remaining muscle tissue and skin from the eyes using scissors and forceps (non-sterile).
NOTE: The size of the scissors and forceps used for the enucleation and cleaning of the eyes depends on the species (e.g., for rat and mouse the instruments are going to be smaller than the ones used for pig and cattle) (see Figure 1).- Wash with PBS and disinfect the eyes by submerging for 2 min in iodine-based solution, rinse with PBS.
- Put one eye on a sterile gauze compress and hold it firmly close to the optic nerve. Open the eye with the scalpel #11 and scissors about 2 mm under the limbus. Remove the anterior segment (cornea, lens, and iris) and put it in a Petri dish. Leave the bulb with the vitreous until RPE cells are isolated.
2. Isolation of IPE cells
- Remove the lens and with fine forceps delicately pull out the iris containing the IPE cells. Place the iris in a Petri dish, wash with sterile PBS and leave it in PBS until more irises are prepared. Remove the ciliary body from the iris by cutting with a scalpel #10. After the preparation of two iris, incubate with 2 mL of 0.25% trypsin at 37 °C for 10 min.
- Remove the trypsin and add 2 mL complete medium to the iris and isolate the cells by carefully scratching with a flat fire-polished Pasteur pipette. Transfer the cell suspension into a 15 mL tube. Centrifuge the cells 10 min at 120 x g. Take 10 µL of the cell suspension and dilute 1:4 with trypan blue to count the cells in the Neubauer chamber.
- If not transfected immediately, seed 320,000 cells/well in a 6-well plate in 3 mL of complete medium (10% FBS) (for seeding see Table 1). Place the plate in an incubator and culture it at 37 °C, 5% CO2.
3. Isolation of RPE cells
- The RPE cells can be isolated by removing vitreous humor and retina from the posterior segment with thin forceps. Avoid damaging the retinal pigment epithelium.
- Place the bulb in a Petri dish and wash with PBS. After preparation of 2 eyes fill the bulb about ¾ with trypsin and incubate for 25 min at 37 °C with the lid of the Petri dish on top of the bulbi.
- Remove the trypsin and add 1 mL complete medium. Using a curved fire-polished Pasteur pipette, carefully remove RPE cells. Make sure to scrape from the bottom to the top to avoid slipping down of the choroid-Bruch's membrane complex. Collect the cell suspension within the bulb using a 1,000 µL pipette and transfer into a 1.5 mL tube for resuspension. Take 10 µL of the cell suspension and dilute 1:8 with trypan blue to count the cells in the Neubauer chamber. Centrifuge the cells 10 min at 120 x g.
- If not transfected immediately, seed 320,000 cells/well in a 6-well plate in 3 mL of complete medium (10% FBS) (for seeding see Table 1). Place the plate in an incubator and culture it at 37 °C, 5% CO2.
Table 1: Number of primary PE cells isolated from eyes from different species.
| Species | Trypsin treatment | N° IPE cells | N° RPE cells | Plate for seeding (100,000 cells/cm2) |
| Mouse/rat | Yes | ~50,000 | ~150,000 | 24-well plates |
| Rabbit | Yes | ~350,000 | ~2,500,000 | 24-well plates |
| Pig | No | ~1,000,000 | ~3,000,000 | 24-well plates |
| Bovine | Yes | ~1,700,000 | ~5,000,000 | 6-well plates |
Representative Results
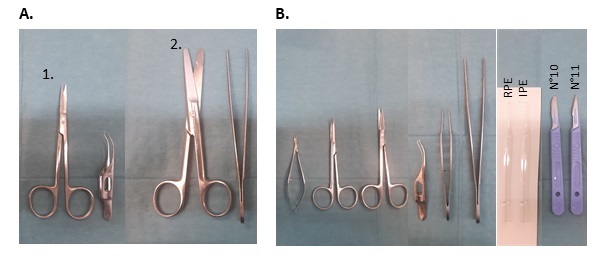
Figure 1: Instruments used for PE isolation depending on the species. (A) Non-sterile instruments used for the enucleation of the eyes and cleaning of remaining muscle tissue and skin. Set 1 is used for rat, mouse, and rabbit, and set 2 is used for pig and cattle eyes. (B) Sterile instruments used for PE isolation. Note the different size of scissors and forceps used depending on the eye size. Round and flat fire-polished Pasteur pipettes are used for the scrape of RPE and IPE, respectively.
Divulgazioni
The authors have nothing to disclose.
Materials
| 6-well plate | Greiner | 7657160 | |
| Betadine | Mundipharma | ||
| Colibri forceps (sterile) | |||
| Forceps (different size) (sterile) | |||
| Gauze compress | PROMEDICAL AG | 25403 | |
| Neubauer chamber | Marienfeld-superior | 640010 | |
| Pasteur pipette (fire-polish) | Witeg | 4100150 | |
| PBS 1X | Sigma-Aldrich | D8537 | |
| Penicillin/Streptomycin | Sigma-Aldrich | P0781-100 | |
| Petri dish | ThermoFisher Scientific | 150288 | |
| DMEM/Ham`s F12 | Sigma-Aldrich | D8062 | |
| FBS | Brunschwig | P40-37500 | |
| Scalpel no. 10 | Swann-Morton | 501 | |
| Scalpel no. 11 | Swann-Morton | 503 | |
| Sharp-sharp tip curved Extra Fine Bonn Scissors (sterile) | |||
| Sharp-sharp tip straight Extra Fine Bonn Scissors (sterile) | |||
| Trypsin 0.25% | ThermoFisher Scientific | 25050014 | |
| Vannas spring scissors curved (sterile) |

