Orthotopic Porcine Kidney Transplantation: A Technique to Transplant Graft Kidney in Porcine Model
Abstract
Source: Liu, W. J. et al., Orthotopic Kidney Auto-Transplantation in a Porcine Model Using 24 Hours Organ Preservation and Continuous Telemetry. J. Vis. Exp. (2020).
This video describes orthotopic kidney auto-transplantation in a porcine model. This surgical procedure involves the transplantation of an animal's kidney graft at its original anatomical location. The model is used to investigate new treatments for end-stage kidney diseases in humans.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Basic techniques and common procedures
- Use female German landrace pigs (or comparable) for this protocol. Figure 1 depicts the summary of the described experimental protocol. Deliver the animals to the research facility 14 days before the surgery for acclimatization and house them in a temperature- and humidity-controlled barrier environment with a 12 h light and dark cycle (Figure 2). Fast the animals overnight before surgery.
- Premedicate by an initial intramuscular injection of azaperone (4 mg/kg) and atropine (0.1 mg/kg), followed by an injection of ketamine (15 mg/kg) 10 min later.
- After premedication, weigh the animal and transfer it directly from the housing facility to the central OR facility anesthesia preparation room.
- Cannulate one of the large ear veins using an 18 G peripheral venous catheter. Monitor the animal by a standard ECG and pulse oximetry.
- Initiate the anesthesia with propofol (3 mg/kg).
- Expose the vocal cord with a laryngoscope and insert a 7.5 mm endotracheal tube. The cuff is blocked with air according to the manufacturer's recommendations.
- Insert an oro-gastric drainage tube to remove fluid and air from the stomach.
- Insert a urinary catheter via the urethra.
- Subsequently, trim the skin in the area of the surgical incision.
- Apply eye ointment to prevent drying of the cornea during surgery.
- After orotracheal intubation, maintain anesthesia with isoflurane (final expiratory 1.45-2.0 Vol.%) and fentanyl (3-7.5 μg/kg/h).
- Ensure active intraoperative temperature control of the animal by a heating pad and using warmed air. Insert a rectal probe to monitor body temperature (target temperature 36.5 °C – 37.5 °C).
- Administer antibiotic prophylaxis using cefuroxime (35 mg/kg i.v.). Infuse Ringer solution at 4 mL/kg/h and increase to 8 mL/kg/h after skin incision. Administer a prophylactic dose of pantoprazole (40 mg i.v.) over the ear vein access.
- Perform all surgical procedures under sterile conditions according to the general principles of surgical asepsis and antisepsis. Disinfect the surgical field with povidone-iodine solution and cover with surgical drapes.
2. Contralateral nephrectomy and orthotopic kidney auto-transplantation
- During the recipient operation, adapt premedication and initial anesthesia to the restricted renal metabolism and avoid the use of ketamine. Induction is performed with propofol (3-5 mg/kg i.v.), midazolam (0,25 mg/kg i.v.), and atropine (0.1 mg/kg i.m.). Thereafter, the preoperative preparation is identical to the procedures described in section 1.
- Maintain anesthesia with isoflurane (final expiratory 1.45-2.0 Vol.%) and fentanyl (3 – 7,5 μg/kg/h) and propofol (2 – 4 mg/kg/h).
- Check and continuously monitor ECG, pulse oximetry, rectal temperature and the function of the telemetry transponder implanted in the right flank.
NOTE: Strict anesthesia and blood pressure control is of crucial importance during the implantation procedure. - In rare cases where the arterial blood pressure signal registered over the telemetry transponder is not satisfactory due to the supine position of the animal, place a further arterial catheter into the right femoral artery using percutaneous puncture and the Seldinger technique.
- Following sterile draping, reopen the median laparotomy and expose the surgical field using the abdominal retractor. The colon and small bowel are placed to the left side of the abdomen to expose the intact right kidney.
- Dissect the contra-lateral (right) kidney and its vessels from the surrounding tissue. Dissect the right renal vein and renal artery in the direction of the kidney hilum to ensure sufficient vessel length for anastomosis.
- Five minutes before vascular clamping, inject natrium-heparin intravenously (100 I.U./kg).
- Clamp the right renal artery and the right renal vein using vascular clamps. The right kidney is removed. The vessels are checked for integrity before starting the anastomoses.
- Place the preserved graft kidney into the abdomen and start the venous and arterial anastomoses.
- From this point onwards, keep the mean arterial pressure over 80-90 mm Hg to ensure good early perfusion of the kidney graft following reperfusion. Achieve this partially by adequate volume management and partially by the administration of norepinephrine (0.1 – 1.0 μg/kg/min as a continuous infusion using the mean arterial blood pressure and heart rate for monitoring the efficiency).
- Perform end-to-end anastomosis of the renal vein:
- After placing two corner stitches using 5-0 polypropylene, suture the back wall in a continuous fashion.
- Tie the cranial corner stitch and tie it together with the thread used for the back wall.
- After finishing the back wall, use the cranial corner stitch to suture the front wall in a cranio-caudal direction. Flush the vein with a heparinized saline solution (100 I.U./mL). Tie the caudal corner stitch.
NOTE: In case of a size mismatch between the donor and recipient sides, a small growth-factor can be used to ensure a wide and sufficient anastomosis. There are many possible variations of the porcine renal vein branches. In the case of complex venous anatomy, a modified anastomosis approach is necessary (see Figure 3).
- Perform the end-to-end anastomosis of the renal artery:
- Use a 6-0 polypropylene cranial corner stitch to perform the arterial anastomosis. Placing a further caudal, supporting corner stitch which is later removed, is optional.
- Suture the back wall in a continuous fashion using the parachute technique. After arriving at the caudal corner remove the second corner stitch (if applicable).
- Suture the front wall with the other end of the double-armed 6-0 polypropylene suture. Flush the artery with a heparinized saline solution (100 I.U./mL). Tie the two threads at the caudal corner.
- Record the time needed for performing both anastomoses with a target warm ischemia time of <40 min.
- Reperfuse the kidney by opening the venous vascular clamp and subsequently the arterial clamp. Check for significant bleeding.
- If no significant bleeding from the anastomoses is observed, unwrap the kidney graft and pour warm normal saline solution in the abdomen covering the reperfused graft.
- Reposition the graft, if needed, to ensure homogeneous reperfusion and avoid congestion.
- Administer papaverine topically to the outside of the renal artery and the arterial anastomosis (5 mL undiluted).
- After reperfusion, infuse 250 mL of 20% glucose solution to induce osmotic dieresis followed by the administration of a single dose of 80 mg of furosemide.
NOTE: Following this, initial urine production may be observed. - To ensure urinary drainage, pass a 12 French pediatric urine catheter through the abdominal wall of the right flank of the animal, retroperitoneally.
- Secure the catheter in the ureter using ligatures (2-0 polyglactin) and block the catheter with 2 mL saline. Further sutures are used to adapt and secure the ureter to the peritoneum of the abdominal wall (2-0 polypropylene). The catheter is also secured to the skin with at least two single knot sutures (2-0 polypropylene).
- Close the peritoneal layer over the kidney to prevent dislocation of the kidney graft and kinking of the vascular anastomoses (3-0 polyglactin).
- Close the abdomen in a similar 4-layer fashion as described earlier for the graft retrieval.
- Following abdominal closure, maintain normothermia on the OR table.
NOTE: Mean arterial blood pressure should be maintained over 80 mm Hg until the animal is awake and is in a prone position. Following abdominal closure, use color Doppler ultrasound to ensure adequate arterial and venous perfusion of the kidney graft (Figure 4). Monitor the animal closely until it is fully awake and can walk and drink spontaneously. The animals are given 1 L of Ringer solution during the recovery phase. Subsequently, return the animal to its box in the housing facility.
Representative Results
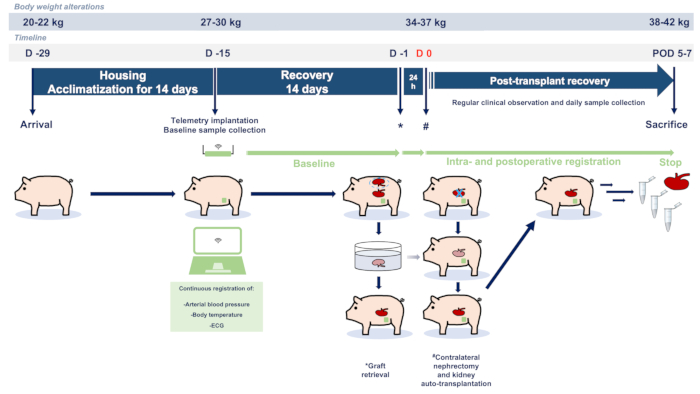
Figure 1: Study flowchart and protocol.
Abbreviations used: POD-postoperative day; ECG-electrocardiography.
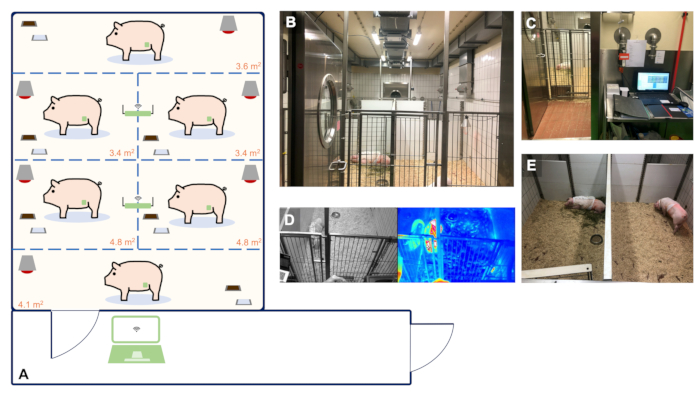
Figure 2: Animal housing facility with real-time and continuous telemetry monitoring of up to 6 animals.
(A) Schematic blueprint of our facility suitable for the housing and telemetry monitoring of up to 6 animals. The size of the single holding boxes was determined based on the guidelines of the EU Directive 2010/63 and ETS 123 Appendix A. Panels A-E show representative images of the organization of our facility. (B) Animal room for the housing of 6 animals. (C) Observation room with a PC used for the continuous registration of telemetry data. (D) Real-time video and thermal footage of the animals. (E) Individual holding ensuring acoustic and olfactory contact of the animals with their companions to avoid social isolation.
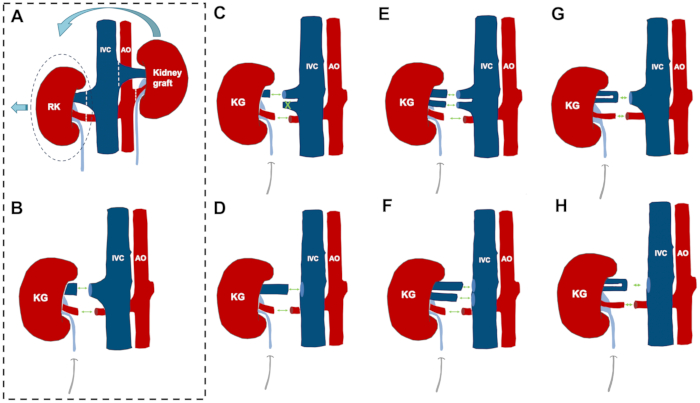
Figure 3: Orthotopic kidney auto-transplantation and anatomical variations and reconstruction possibilities.
(A, B) The steps of the orthotopic kidney auto-transplantation model in case of a "standard" vascular anatomy. (C) Variation 1: while one larger vein comes with the donor kidney, there are two veins on the recipient side. Management: the smaller vein is closed by a ligature and the anastomosis is performed end to end between the renal veins. (D) Variation 2: while one larger vein comes with the donor kidney, there is no suitable recipient vessel on the contralateral side (e.g., size mismatch). Management: end to side anastomosis of the renal vein to the inferior vena cava. (E) Variation 3: two similar-sized veins on both sides. Management: reconstruction by two venous anastomoses. (F) Variation 4: while two similar-sized veins come with the donor kidney, there is no suitable recipient vessel on the contralateral side. Management: end to side anastomosis of the renal vein to the inferior vena cava in the case of two renal veins. (G) Variation 5: a donor kidney comes with a vein showing an early bifurcation, while there is one large vein on the contralateral side. Management: end-to-end anastomosis of the short common channel of the donor renal vein with one large vein on the recipient side. (H) Variation 6: while the donor kidney comes with a single renal vein with an early bifurcation, there is no suitable recipient vessel on the contralateral side. Management: end to side anastomosis of the short common channel of the donor renal vein to the inferior vena cava. This figure depicts a handful of the more frequent variations and is not statistically comprehensive in terms of all variations possible in German landrace pigs. Abbreviations used: KG-kidney graft; RK-right kidney; IVC-inferior vena cava; AO-aorta.
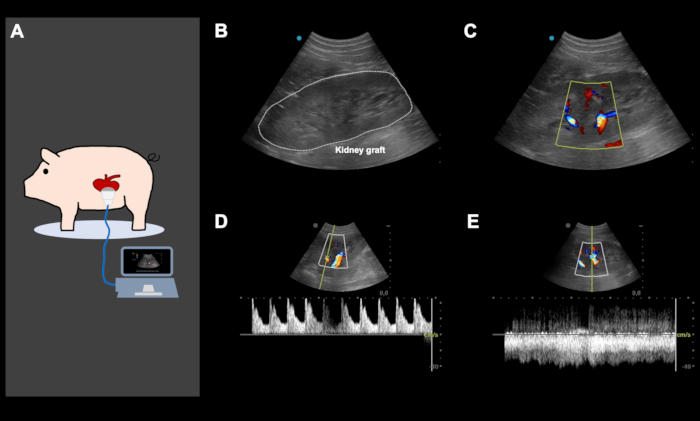
Figure 4: Representative color Doppler ultrasound images, directly after orthotopic kidney auto-transplantation and abdominal closure.
(A) Color Doppler ultrasound is performed directly following the implantation of the kidney and abdominal closure, to ensure good arterial and venous perfusion of the kidney graft and to screen for potential iatrogenic vascular kinking. Ultrasound was also used daily and on-demand, based on the clinical performance of the animal to screen for various problems. (B-E) Representative ultrasound images of a kidney graft following implantation. The image of the kidney graft with and without color Doppler (B, C) shows an excellent arterial (D) and venous perfusion (E). This figure shows representative images from the same animal.
Divulgazioni
The authors have nothing to disclose.
Materials
| Anesthesia materials, drugs and medications | |||
| Atropine sulfate solution for injection, 100mg | Dr. Franz Köhler Chemie GmbH, Bensheim, Germany | 1821288 | parasympatholytic agent, premedication |
| Bepanthen ointment for eyes and nose | Bayer Vital AG, Leverkusen, Germany | 1578675 | eye ointment |
| BD Discardit II syringes, 2ml, 5ml, 10ml,20ml | Becton Dickinson GmbH, Heidelberg, Germany | 300928, 309050,309110, 300296 | syringes |
| BD Micolance 3 (20G yellow) Cannula | Becton Dickinson GmbH, Heidelberg, Germany | 305888 | venous catheter |
| BD Venflon Pro Safety (20G pink) | Becton Dickinson GmbH, Heidelberg, Germany | 4491101 | venous catheter |
| Buprenorphine (Buprenovet) | Bayer Vital AG, Leverkusen, Germany | 794-996 | analgesia |
| Cefuroxime 750mg, powder for preparing injection solution | FRESENIUS KABI Deutschland GmbH, Bad Homburg, Germany | J01DC02 | antibiotics |
| Covidien Hi-Contour, Endotracheal Tube 7,5 with Cuffed Murphy Eye | Covidien Deutschland GmbH,Neustadt/Donau, Germany | COV-107-75E | endotracheal Tube |
| FENTANYL 0,5 mg Rotexmedica solution for injection Rotexmedica GmbH | Arzneimittelwerk, Trittau, Germany | 4993593 | opioide analgetic agent |
| Furosemide-ratiopharm 250 mg/25 ml solution for injection | Ratiopharm GmbH, Ulm, Germany | 1479542 | loop diuretics |
| Glucose 5% solution for infusion (500ml, 250ml) | B. Braun Deutschland GmbH & Co. KG, Melsungen, Germany | 3705273, 03705422 | infusion fluid |
| Glucose 20% solution for infusion | B. Braun Deutschland GmbH & Co. KG, Melsungen, Germany | 4164483 | osmotic diuresis |
| Heparin-Sodium 5000 I.E./ml | B. Braun Deutschland GmbH & Co. KG, Melsungen, Germany | 15782698 | anticoagulant |
| Isoflurane-Piramal (Isoflurane) | Piramal Critical Care Deutschland GmbH, Hallbergmoos, Germany | 9714675 | volatile anaesthetic agent |
| Ketamine (Ketamine hydrochloride) 10% | Medistar Arzneimittelvertrieb GmbH, Ascheberg, Germany | 4230 | general anaestetic agent |
| MIDAZOLAM 15mg/3ml | Rotexmedica GmbH, Arzneimittelwerk, Trittau, Germany | 828093 | hypnotica, sedative agent |
| NaCl 0,9% solution for infusion (500ml,1000ml) | B. Braun Deutschland GmbH & Co. KG, Melsungen, Germany | 864671.8779 | infusion fluid |
| Norepinephrine (Arterenol) | Sanofi-Aventis Deutschland GmbH, Frankfurt, Germany | 16180 | increase in blood pressure |
| Pantoprazole 40mg/solution for injection | Laboratorios Normon,Madrid, Spain | 11068 | proton pump inhibitor |
| Paveron N 25mg/ml solution for injection (Papaverine Hydrochloride) | LINDEN Arzneimittel-Vertrieb-GmbH, Heuchelheim, Germany | 2748990 | spasmolytic agent for vasodilatation |
| Propofol 1% (10mg/ml) MCT Fresenius | FRESENIUS KABI Deutschland, GmbH, Bad Homburg, Germany | 654210 | general anaesthetic agent |
| Ringer solution | B. Braun Deutschland GmbH & Co. KG, Melsungen, Germany | 1471411 | infusion fluid |
| Urine catheter ruffle 12CH | Wirutec Rüsch Medical Vertriebs GmbH, Sulzbach, Germany | RÜSCH-180605-12 | transurethral urine catheter |
| Surgical materials | |||
| Appose ULC Skin Stapler | Covidien Deutschland GmbH,Neustadt/Donau, Germany | 8886803712 | skin stapler |
| Feather Disposable Scapel (11)(21) | Feather, Japan | 8902305.395 | scapel |
| Prolene 2-0, blue monofil VISIBLACK, FS needle | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | EH7038H | skin |
| Prolene 5-0 (simply angulated, C1 needle) blue monofil VISI-BLACK | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | EH7227H | vascular |
| Prolene 5-0 (double armed, C1 needle) 60cm | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | KBB5661H | vascular |
| Prolene 6-0 (double armed, C1 needle) 60cm | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | EH7228H | vascular |
| Sempermed derma PF Surgical Gloves Seril Gr. 7, 7.5, 8 | Semperit investment Asia Pte Ltd, Singapore | 4200782, 4200871, 4200894 | surgical gloves |
| Sentinex® PRO Surgical Gowns Spunlace XL 150cm | Lohmann & Rauscher GmbH & Co. KG, Neuwied, Germany | 19302 | surgical gown |
| Telasorp Belly wipes (green 45x45cm) | PAUL HARTMANN AG, Heidenheim, Germany | 4542437 | abdominal towel |
| Pediatric urine catheter | Uromed Kurt Drews KG, Oststeinbeck, Germany | PZN 03280856 | used for the uretero-cutaneus stoma |
| VICRYL- 0 MH Plus | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | V324 | fascial closure |
| VICRYL – 3-0, SH1 Plus needle, 75cm | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | W9114 | subcutaneous suture, peritoneal suture |
| VICRYL – 3-0, SH1 Plus needle, 75cm | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | V780 | subcutaneous suture, peritoneal suture |
| VICRYL – ligatures Sutupak purple braided, 3-0 | Johnson & Johnson Medical GmbH- Ethicon Deutschland, Norderstedt, Germany | V1215E | threads for ligature |
| 3M™ Standard Surgical Mask 1810F | 3M Deutschland GmbH, Neuss, Germany | 3M-ID 7000039767 | surgical mask |
| Surgical instruments | |||
| Anatomical forceps Standard | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | PZ0260 | anatomical forceps |
| Atraumatic tweezers steel, De Bakey Tip 1,5mm 8" | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | GF0840 | anatomical atraumatic forceps |
| Bipolar forceps 16 cm straight, Branch 0,30 mm pointed, universal fit | Bühler Instrumente Medizintechnik GmbH,Tuttlingen, Germany | 08/0016-A | biopolar forceps |
| Bulldog clamp atraumatic, curved, De bakey 78 mm, 3" | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | GF0900 | bulldog clamps |
| DE BAKEY-SATINSKY vascular clamp 215mm | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | GF1661 | vascular clamp |
| Dissecting scissors Mayo, 250 mm, 10" | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | SC2232 | Scissors for dissection |
| Dissecting scissors Metzenbaum-Fino, 260 mm, 101/4" | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | SC2290 | Scissors for dissection |
| Draeger CATO Anesthetic machine with PM8050 Monitor | Dräger, Drägerwerk AG & Co. KGaA, Lübeck, Germany | 106782 | Ventilation System |
| Fine Tweezers, ADSON 180 mm | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | ADSONPZ0571 | fine forceps |
| Gosset abdomenal wall spreader | CHIRU-INSTRUMENTE, Kaierstuhl, Germany | 09-621512 | abdominal retractor |
| Microsurgical/watermaker tweezers LINZ 150mm 6" Ergo round handle | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | MN0087 | fine microsurgical forceps |
| Surgical forceps Standard 5 3/4" | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | PZ1260 | surgical forceps |
| Surgical scissors standard pointed-blunt (thread/cloth scissors)175 mm, 7" | ASANUS Medizintechnik GmbH, Tuttlingen, Germany | SC1522 | surgical scissors |
| Further material | |||
| Heating pad | Eickemeyer – Medizintechnik für Tierärzte KG, Tuttlingen, Germany | 648050 MHP-E1220 | maintain body temperature during surgery |
| Laryngoscope, customized | Wittex GmbH, Simbach, Germany | 333222230 | expose the vocal cord |
| Rectal temperature probe | Asmuth Medizintechnik, Minden, Germany | ASD-RA4 | measure body temperature |
| Ultrasound device, Sonosite Edge-II | FUJIFILM SonoSite GmbH, Frankfurt, Germany | V21822 | ultrasound and color Doppler |
| Urine bag 2000ml Volume | ASID BONZ GmbH, Herrenberg, Germany | 2062578 | disposable urine bag connected to the uretero-cutaneous fistula catheter |

