Binary Bacterial Artificial Chromosome Vector Based Gene Transformation in Arabidopsis Plants: A Technique to Generate Transgenic Plants With Single-Copy Insertion
Abstract
Source: Tark-Dame, M., et al. Generating Transgenic Plants with Single-copy Insertions Using BIBAC-GW Binary Vector. J. Vis. Exp. (2018).
In this video, we demonstrate generation of transgenic Arabidopsis plants expressing stable transgene through Agrobacterium-mediated binary bacterial artificial chromosome-based gene transformation.
Protocol
1. Arabidopsis Transformation
- Prepare the Arabidopsis plants for transformation.
- Grow Arabidopsis plants in a greenhouse or climate-controlled growth chamber until they are flowering (12 pots with 9 plants each per dipping).
- Clip the first bolts to allow more secondary bolts to emerge. Plants are ready for dipping 4–6 days after clipping, when the plants have many immature flower heads and not many fertilized siliques.
- Floral dipping
- Dip inflorescences for 5–10s in Agrobacterium suspension carrying T-DNA cloned with binary bacterial artificial chromosome or BIBAC. Use gentle agitation.
- Wrap the above-ground parts of the plants in cling film to keep the humidity high, and cover the plant pots with a box to keep the plants in the dark. Incubate the plants for 2 days in a greenhouse/growth chamber.
- Remove the box and the cling film and grow the plants to maturity in a greenhouse/growth chamber.
NOTE: To increase the efficiency of transformation, the same plants can be re-dipped 7 days after the first dipping. - Harvest the seeds. Pool and analyze the seeds (T1) of plants, transformed with the same construct, as a single set.
- Screen for transgenic plants.
- To screen for transgenic plants transformed with a pBIBAC-RFP-GW derivative, analyze the seeds using fluorescence microscopy. In order to detect DsRed expression in seed coats, image the seeds at an excitation of 560 nm and emission of 600–650 nm. Separate the fluorescent seeds from non-fluorescent counterparts using forceps.
- To screen for transgenic plants transformed with a pBIBAC-BAR-GW derivative, sow the seeds in trays filled with soil (~2,500 seeds/0.1 m2). To ensure an even spreading of seeds over trays, suspend seeds in 0.1% agar in 0.5x Murashige Skoog medium (MS), and spread the seeds using a 1 mL pipette.
NOTE: To stimulate the seeds to germinate in a synchronous manner, incubate the seeds for at least 2 days at 4 °C. This can be done before or after sowing the seeds.- Spray the seedlings with 0.5% Glufosinate-ammonium solution 2 weeks and 3 weeks after sowing in trays. Use 500 mL of Glufosinate-ammonium solution per 1 m2.
- Transfer surviving seedlings to individual pots. A typical image of a tray with seedlings before and after (second) Glufosinate-ammonium treatment are shown in Figure 1.
- Analyze the Glufosinate-ammonium-resistant plants by PCR for the presence of the construct of interest.
- Isolate the genomic plant DNA for PCR.
Representative Results
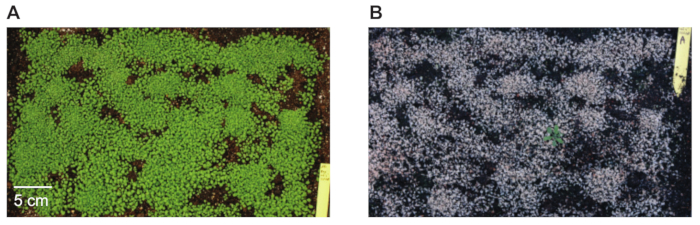
Figure 1: Tray filled with Arabidopsis seedlings before and after Glufosinate-ammonium treatment. Seedlings not expressing the bar gene that is present in the pBIBAC-BAR-GW T-DNA die after being sprayed with Glufosinate-ammonium solution. The photos show the same tray of seedlings (A) before spraying with Glufosinate-ammonium, 14 days after sowing, and (B) 10 days later, after being sprayed twice.
Divulgazioni
The authors have nothing to disclose.
Materials
| Kanamycin sulphate monohydrate | Duchefa | K0126 | |
| Gateway LR clonase enzyme mix | Thermo Scientific – Invitrogen | 11791-019 | |
| Murashige Skoog medium | Duchefa | M0221 | |
| Agar | BD | 214010 | |
| Glufosinate-ammonium (Basta) | Bayer | 79391781 | |
| Phosphor imager | GE Healthcare Life Sciences | Typhoon FLA 7000 | |
| cling film (Saran wrap) | Omnilabo | 1090681 | |
| Agarose | Thermo Scientific – Invitrogen | 16500 |

