Three-Chambered Array-Based Impedance Assay: A Real-Time Analysis Technique to Assess Invasive Potential of Cancer Cells by Measuring Electrical Impedance
Abstract
Source: Sharif, G. M. et al., Real-Time Detection and Capture of Invasive Cell Subpopulations from Co-Cultures. J. Vis. Exp. (2022).
This video describes an invasion assay using a three-chambered array based on electrical impedance. This technique measures the invasive potential of cancer cells under the influence of soluble factors secreted by the resident stromal cells in tumor microenvironments.
Protocol
1. New chamber design (Figure 1)
- Open a new dual-chamber cell analyzer plate. Set aside the top chamber with electrodes.
- Using a milling machine, shave off 2 mm of the U-shaped bottom wells of the cell analyzer plate.
- Attach a 2 cm x 7 cm polyethersulfone (PES) membrane with 0.2 μm pore size to the bottom of the shaved wells using UV-curated adhesive. Allow 30 min curation time to ensure the glue is completely cured and inert.
- Using a milling machine, cut out two longitudinal slits (1.5 mm x 5.6 mm) along the sides to snap into the ridges of the newly fabricated third chamber.
- Using a milling machine, create a third polycarbonate chamber that replicates the overall dimensions of the cell analyzer plate; 72 mm x 18 mm (Table of Materials).
- Create wells 4.8 mm deep and 4.75 mm in diameter to replicate the 16-well design of the cell analyzer plate. This allows 90 μL of volume per well.
- On the sides, create two triangular ridges so that the chamber locks into the original slits created in step 1.4. The horizontal part of the triangle is 1.5 mm, the vertical is 1.4 mm, and the hypotenuse is 2.052 mm.
- Create a knob on the short side that is 50.8 mm in diameter and 1.397 mm in height to fit into the original plate's notch (Figure 1, Middle).
- Use a 0.9 mm thick rubber washer for each well to provide a sealed fit.
2. Cell culture (MDA-MB-231, DCIS, DCIS-Δ4, J2-fibroblasts)
- Wash adherent cell cultures (~70% confluence) with 1x phosphate-buffered saline (PBS).
- Add 0.05% trypsin-EDTA solution to lift the cells off.
- Neutralize the trypsin solution with cell culture media containing serum and count the cells using an aliquot of the cell suspension.
NOTE: The specific cell culture media can be found in Table 1.
3. Patient-derived xenograft dissociation
- Chop a fresh tumor piece (1 cm2) into fine mush using a sterile scalpel.
- Place in a 50 mL conical tube with 20 mL of DMEM F12 media supplemented with 3 mg/mL trypsin and 2 mg/mL collagenase.
- Incubate in a thermal shaker (150 RPM) at 37°C for 20 min.
- Spin the tube at 500 x g for 5 min; remove the supernatant.
- Add 20 μL of DMEM F12 + 2% FBS to wash the cells; spin at 300 x g for 5 min and remove the supernatant. Repeat the wash two more times.
- Resuspend in 1 mL of PDX media (Table 1) to count the cells.
4. Bone marrow cell extraction
- Flush the bone marrow (BM) collection filter with 25 mL of 1x PBS.
NOTE: In this study, PBS was added to a used BM collection filter from the hospital to collect the remaining BM in the filter. - Add the flushed BM slowly to a 50 mL conical tube with 25 mL of density gradient medium, taking care to keep the layers as separate as possible.
- Spin at 800 x g for 20 min at 18 °C.
- Siphon off the top layers after centrifugation (fat/plasma) and transfer 5 mL of the white layer above the density gradient medium that has the BM cells to a 15 mL conical tube.
NOTE: Alternatively, dip a 5 mL pipette into the top layer until it touches the middle layer (BM), and pipette out the middle layer very slowly without moving the pipette. - Fill the 15 mL conical tube with 1x PBS (~10 mL) and spin at 300 x g for 15 min.
- Remove the supernatant; the remaining white pellet is the BM.
- If red blood cells are observed in the pellet, add 5 mL of RBC lysis solution (Table of Materials) and let it sit for 5 min at room temperature (RT). Spin at 300 x g for 5 min and remove the supernatant.
- Add 10 mL of 1x PBS to wash the cells, spin at 300 x g for 5 min, and remove the supernatant. Repeat the RBC lysis (step 4.7) until the pellet is white.
5. Cell seeding and assembly
- Place all three sterile chambers in the tissue culture hood.
- Locate the knob on the short side of the lower chamber. Orient the lower chamber so that the knob is facing the experimenter.
- Add 30,000-50,000 cells in 90 μL of media to each well of the lower chamber. Avoid forming bubbles. These are the stromal cells that will provide secreted factors but will not be detected by the electrodes of the top chamber.
- Use 5% fetal bovine serum-supplemented media in two lower chamber wells as a positive control for cell motility. Use 0% serum-supplemented media as a negative control.
- Let the lower chamber with the cells sit for 10-15 min in the hood to settle.
NOTE: This step is recommended if cells are adherent or grow in suspension. - Rotate the lower chamber at 90° and place the middle chamber on top so that the knob on the lower chamber slides into the notch on the middle chamber.
NOTE: The knob on the lower chamber and the blue dot on the middle chamber are at opposite ends of the assembly. - Push vertically down until a click sound is heard from each of the long sides of the assembly.
- Add 160 μL of serum-free media to all the wells of the middle chamber.
- Make sure a dome-shaped meniscus is visible after the wells are filled; otherwise, adjust the final volume based on the pipette calibration. Avoid forming bubbles.
- Place the top chamber with electrodes facing down onto the middle chamber making sure to align the blue dots on the middle and top chambers.
- Push vertically down until a click sound is heard from each of the long sides of the assembly.
- Add 25-50 μL of serum-free media to the top chamber.
- Mount the assembly on the dual-purpose cell analyzer in the tissue culture incubator and wait for 30 min before measuring the background.
NOTE: This time is necessary to equilibrate the array and can be used to prepare the cell lines to be added to the top chamber. - Measure the background (see section 6) and place the assembly back into the tissue culture hood.
- Add 30,000-50,000 cells in 100 μL of serum-free media to each well of the top chamber. These are the cells that the electrode will detect once they successfully migrate through the membrane
NOTE: To achieve maximum response, it is recommended to grow cells in serum-free or low-serum media for 6-18 h before performing the assay. - Let the assembly stand in the hood for 30 min before mounting on the dual-purpose cell analyzer for impedance measurement.
6. Background and impedance measurement
- Place the array into the cradle of the dual-purpose cell analyzer instrument.
- Open the cell analyzer software and select the cradle to be used.
- Click on the Message tab and make sure it says Connections OK to ensure the array is well placed in the cradle and the electrodes are well aligned with the sensors.
- Click on the Experiment Notes tab and fill in as much information about the experiment as possible.
- Click on the Layout tab and fill in the description of the array layout.
- Click on the Schedule tab and add two steps from the Steps menu; a background step (one sweep) and a test step with 100 sweeps-a sweep every 15 min, totaling 25 h.
- After the array has been in the dual-purpose cell analyzer incubator for 30 min, click on the Play button to start background measurement. A window asking to choose the folder to save the data will pop up.
- After the background measurement is done, remove the array from the cradle and place it back in the cell culture hood.
- Add cells to the top chamber as described in step 5.13, and keep the assembly in the tissue culture hood for 30 min for the cells to settle.
- Place the array back into the dual-purpose cell analyzer and check the Message tab for the Connections OK message.
- Click on the Play button to start impedance measurement.
- Click on the Plot tab to monitor the progress of the signal.
- If the endpoint is reached before 25 h, click on the Abort step from the Execute drop-down menu.
- To export data, right-click on the graph, choose Copy in the list format and then paste the data in a spreadsheet.
NOTE: The data can be exported as cell index or delta cell index. Graph and/or layout information can also be chosen for export.
Table 1: Cell culture media composition. The table lists the compositions of MDA-MD-231 media, J2 Fibroblasts media, DCIS media, and PDX media.
| Media | Constituents | Concentration/proportion |
| MDA-MD-231 media | DMEM | |
| Fetal Bovine Serum (FBS) | 10% | |
| J2 Fibroblasts media | DMEM | |
| Fetal Bovine Serum (FBS) | 10% | |
| DCIS media | DMEM F12 | |
| Horse serum (HS) | 5% | |
| Epidermal growth factor (EGF) | 20 ng/mL | |
| Insulin | 10 μg/mL | |
| Hydrocortisone | 0.5 μg/mL | |
| Cholera toxin | 100 ng/mL | |
| PDX media | DMEM F12 | |
| Fetal Bovine Serum (FBS) | 2% | |
| HEPES | 1 M | |
| Insulin Transferrin Selenium Ethanolamine (ITS) | 10 μg/mL | |
| Hydrocortisone | 0.5 μg/mL | |
| Bovine serum albumin (BSA) | 1 mg/mL |
Representative Results
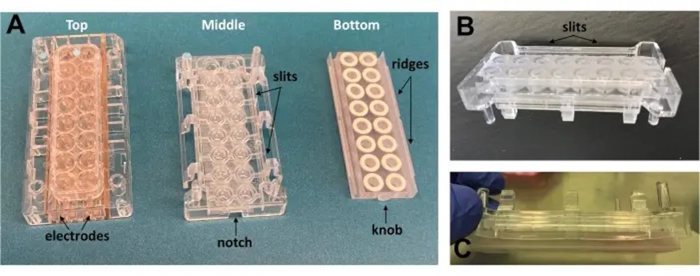
Figure 1: Images of the array chambers and modifications.
(A) The three chambers used to build the array. No modification was made on the top chamber harboring the electrodes. (B) From the middle chamber wells, a height of 2 mm has been shaved off and a membrane attached to the open bottom; longitudinal slits (1.5 mm x 5.6 mm) were added to each side. (C) Lower chamber (72 mm x 18 mm) fabricated to replicate the 16-well design; wells are 4.8 mm deep, and 4.75 mm in diameter, triangle ridges (1.5 mm horizontal x 1.4 mm vertical) are added along the sides to click into the middle chamber slits.
Divulgazioni
The authors have nothing to disclose.
Materials
| 0.05% Trypsin-EDTA | Thermofisher | 25300-054 | |
| Adhesive | Norland Optical Adhesive | NOA63 | |
| Bovine serum albumin (BSA) | Sigma | A9418 | |
| Cell lifter | Sarstedt | 83.1832 | |
| Cholera Toxin from Vibrio cholerae | Thermofisher | 12585-014 | |
| CIM-plate | Agilent 5665817001 | Cell analyzer plate | |
| Collagenase from Clostridium histolyticum | Sigma | C0130 | |
| DMEM | Thermofisher | 11995-065 | |
| DMEM-F12 | Thermofisher | 11875-093 | |
| Fetal Bovine Serum (FBS), Heat Inactivated | Omega Scientific | FB-12 | |
| HEPES | Thermofisher | 15630106 | |
| Horse serum (HS) | Gibco | 16050-122 | |
| Human EGF | Peprotech | AF-100-15 | |
| Hydrocortisone | Sigma | H4001 | |
| Insulin Transferrin Selenium Ethanolamine (ITSX) (100x) | Thermofisher | 51500056 | |
| Insulin, Human Recombinant, Zinc Solution | Sigma | C8052 | |
| J2 Fibroblasts | Stemcell (RRID:CVCL_W667) | 100-0353 | |
| LymphoPrep | Stemcell | 7851 | Density gradient medium for the isolation of mononuclear cells |
| Matrigel | Corning | 354230 | Basement membrane matrix |
| MCFDCIS.com cells ( DCIS) | RRID:CVCL_5552 | ||
| MDA-MB-231 cells | RRID:CVCL_0062 | ||
| Milling machine | Bridgeport Series 1 Vertical | ||
| Phosphate-buffered saline (1x) | Thermofisher | 10010049 | |
| Polyethersulfone (PES) membrane | Sterlitech | PCTF029030 | |
| RBC lysis solution | Stemcell | 7800 | |
| RTCA DP analyzer | Agilent | 3X16 | Dual purpose cell analyzer |
| Trypsin | Sigma | T4799 |

