Abstract
Source: Bokobza, C. et al., Magnetic Isolation of Microglial Cells from Neonate Mouse for Primary Cell Cultures. J. Vis. Exp. (2022)
In this video, we describe a protocol for isolating CD11b cells, including microglia, from mouse pup brains using magnetic-activated cell sorting. The brain tissues are homogenized to obtain single-cell suspensions, which are incubated with anti-CD11b antibody-coated microbeads, followed by a magnetic column-based separation to purify the CD11b+ cell population.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Brain dissociation and magnetic microglial isolation
NOTE: All cell manipulations and resuspensions must be performed with a 1,000 µL pipette with great caution. Applying a high mechanical action may activate or kill microglia cells.
- Transfer 12 brain pieces (~1.2 g) per dissociation tube containing dissociation mixture according to Table 1. For 24 pups, four C-Tubes will be needed (Figure 1A-B).
- Place C-Tubes on the dissociator (with the heating mode). Start the optimized NTDK program in the equipment according to the manufacturer's instructions (Figure 1D).
- Centrifuge at 300 x g for 20 s (at 4 °C) to collect all the cells. Complete the mechanical dissociation by pipetting three times up and down with a 1,000 µL pipette (Figure 1E).
- Transfer the cells to four 15 mL tubes + strainers. Rinse the strainers with 10 mL of 1x Hanks' Balanced Salt Solution (HBSS) with Ca2+ and Mg2+ (HBSS+/+) (Figure 1F).
- Centrifuge at 300 x g for 10 min (at 4 °C) and remove the supernatant. Carefully resuspend the pellet with 10 mL of HBSS+/+ (Figure 1G).
- Centrifuge at 300 x g for 10 min (at 4 °C) and remove the supernatant. Carefully resuspend the pellet with 6 mL of sorting buffer (1x PBS + 0.5% BSA) (Figure 1H).
- Centrifuge at 300 x g for 10 min (at 4 °C) and remove the supernatant. Add 200 µL of CD11b-microbead solution per tube and resuspend carefully (Figure 1I).
- Incubate the tubes for 15-20 min at 4 °C. Carefully resuspend the pellet with 6 mL of sorting buffer (Figure 1I-J).
- Centrifuge at 300 x g for 10 min (at 4 °C) and remove the supernatant. Carefully resuspend the pellet with 8 mL of sorting buffer (Figure 1K).
- Follow the POSSEL program on the separator (see Table of Materials) to prepare eight columns. Transfer cells 1 mL by 1 mL on the column. Wait for all the cells to pass through before adding another mL. Elute CD11b+ cells on a sterile elution plate with 1 mL of sorting buffer (Figure 1L).
- Pool CD11b+ cells in a new 50 mL tube (Figure 1M).
- Centrifuge at 300 x g for 10 min (at 4 °C) and remove the supernatant. Carefully resuspend the pellet with 10 mL of cold microglia medium (macrophage serum-free culture medium (SFM) + 1% of Penicillin-Streptomycin (P/S)) (Figure 1N).
- Count the CD11b+ cells. At P8, one should obtain ~650,000 cells per brain.
NOTE: In the present protocol, the cells were counted using an automated cell counter (see Table of Materials). - Resuspend the cells in cold microglia medium to a final concentration of 650,000-700,000 cells/mL and dispense in cell culture plates.
NOTE: The 6-well plates are for Western Blot (2 mL per well); the 12-well plates are for RT-qPCR analysis (1 mL per well), and the 96-well plates are for phagocytic assay (250 µL per well).
Table 1: Preparation of the dissociation mixture.
| Solution | For one C-Tube (μL) | For four C-tubes (μL) |
| Buffer X | 2850 | 11400 |
| Enzyme P (Papain) | 75 | 300 |
| Enzyme A (DNAse) | 15 | 60 |
| Buffer Y | 30 | 120 |
| Total | 2970 | 11880 |
Representative Results
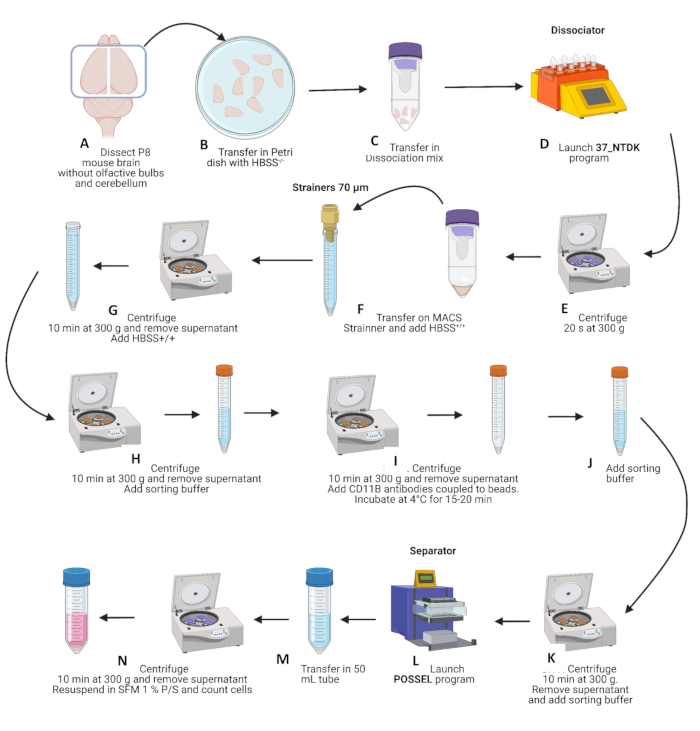
Figure 1: Schematic representation of brain dissociations and microglial cells' isolation. (A-C) After P8 mouse brain dissection and removing olfactive bulbs and cerebellum, brains were first transferred to a Petri dish with HBSS+/+, and then to dissociation tubes containing the dissociation mixture. The C-tubes were placed on the dissociator (with the heating mode), and the NTDK program was started (D). (E) At the end of the program, tubes were centrifuged at 300 x g for 20 s at 4 °C; dissociation was then completed by pipetting three times up and down with a 1,000 µL pipette. (F) Cells were then transferred to 15 mL tubes + strainers of 70 µm and rinsed with 10 mL of HBSS+/+. (G) Samples were then centrifuged at 300 x g for 10 min at 4 °C, the supernatant was removed, and the pellet was resuspended with 10 mL of HBSS+/+. (H) Tubes were again centrifuged at 300 x g for 10 min at 4 °C; the supernatant was removed, and then the pellet was resuspended in 6 mL of sorting buffer. (I) Tubes were centrifuged at 300 x g for 10 min at 4 °C, the supernatant was removed, and the CD11b-microbead (200 µL) solution was added. Tubes were incubated for 15-20 min at 4 °C, and then resuspended in 6 mL of sorting buffer (J) and centrifuged at 300 x g for 10 min at 4 °C. (K) The supernatant was removed, and the pellet was carefully resuspended in 8 mL of sorting buffer. (L) Then, launch the POSSEL program on the separator to prepare columns. Cells were transferred 1 mL by 1 mL on the column, and CD11b cells were eluted on a sterile elution plate with 1 mL of sorting buffer. (M) CD11b cells were pooled in a new 50 mL tube and centrifuged at 300 x g for 10 min at 4 °C, and the supernatant was removed. (N) The pellet was carefully resuspended in 10 mL of cold microglia medium in the last step. The cells were counted and diluted in the microglial medium to a final concentration of 650,000-700,000 cells/mL dispensed in cell culture plates.
Divulgazioni
The authors have nothing to disclose.
Materials
| Bovine Serum Albumin | Miltenyi Biotec | 130-091-376 | |
| CD11b (Microglia) MicroBeads, h, m | Miltenyi Biotec | 130-093-634 | |
| D-PBS (10x) | Thermo Scientific | 14200067 | |
| Falcon Cell culture 12-well plate, flat bottom + lid | Dutscher | 353043 | |
| Falcon tubes 50 mL | Falcon tubes 50 mL | 352098 | |
| Neural Tissue Dissociation Kit – Papain | Miltenyi Biotec | 130-092-628 | |
| Nucleocounter NC-200 | Chemometec | ||
| Nun EZFlip Top Conical Centrifuge Tubes | Thermo Scientific | 362694 | |
| OPTILUX Petri dish – 100 x 20 mm | Dutscher | 353003 | |
| gentleMACS C Tubes (4 x 25 tubes) | Miltenyi Biotec | 130-096-334 | |
| gentleMACS Octo Dissociator with Heaters | Miltenyi Biotec | 130-096-427 | |
| Hanks' Balanced Salt Solution (HBSS) +CaCl2 +MgCl2 10x | Thermo Scientific | 14065049 | |
| Penicillin-streptomycin (10 000 U/mL) | Thermo Scientific | 15140122 | |
| Macrophage-SFM serum-free medium | Thermo Scientific | 12065074 | |
| MACS BSA Stock Solution | Miltenyi Biotec | 130-091-376 | |
| MACS SmartStrainers (70 μm), 4 x 25 pcs | Miltenyi Biotec | 130-110-916 | |
| MultiMACS Cell24 Separator | Miltenyi Biotec | ||
| Multi-24 Column Blocks | Miltenyi Biotec | 130-095-691 |

