The Photoconversion Technique for Exploring Inflammatory Cell Dynamics in Insect Pupae
Abstract
Source: Weavers, H. et al., Long-term In Vivo Tracking of Inflammatory Cell Dynamics Within Drosophila Pupae. J. Vis. Exp. (2018)
The video demonstrates the use of photoconversion to study inflammatory cell dynamics in Drosophila pupae. Green hemocytes are drawn to the wounded epithelium, followed by distant migration. Photoconversion shifts certain hemocytes from green to red, facilitating their movement tracking.
Protocol
This protocol consists of four main sequential steps: (1) Preparation of Drosophila stocks and staging of Drosophila pupae, (2) Pupal dissection and mounting, (3) Pupal wounding, (4) in vivo time-lapse confocal imaging.
1. Preparation of Drosophila Stocks and Staging of Pupae
- Obtain appropriate Drosophila stocks (see Introduction and Table of Materials).
- Collect young healthy adult flies of the appropriate genotype.
- Select adult flies by using carbon dioxide gas pads to briefly anesthetize the flies and a fine paintbrush to transfer flies of the appropriate genotype or gender to a collection vial.
- Add 20 virgin females and 20 males to each vial containing standard fly food media (a cornmeal-molasses-agar mixture, see Table of Materials) supplemented with yeast.
- For optimal pupal generation, tip the adult flies each day onto new food in fresh vials, keeping all vials at 25 °C.
NOTE: If the Gal4-upstream activating sequence (Gal4-UAS) system is being used to drive lineage-specific gene expression, all steps should be performed at 25 °C or above, as the Gal4-UAS system is temperature sensitive. - 18 h before the scheduled imaging session, select at least 10 newly formed white pre-pupae (Figure 1A) from the vials (i.e. 0 h after puparium formation, APF) using forceps or a fine paintbrush to dislodge pupae from the interior vial surface and transfer pupae carefully to the side of a clean empty plastic vial.
NOTE: Wandering 3rd instar larvae crawl upwards out of the food medium to undergo pupation; newly formed white prepupae are easily identified as they possess everted anterior spiracles and are stationary (unlike 3rd instar larvae). The cuticle transforms into the 'puparium' (the pupal case) which is initially soft and white. Care must be taken to avoid damaging the pupae, as this can lead not only to an unwanted injury-induced inflammatory response but also to a significant developmental delay. - Age the selected pupae to the appropriate developmental stage (18 h APF will give optimal results) in the vial at 25 °C.
NOTE: As the pupae develop, the pupal case will become progressively darker and more brittle. - Prepare other reagents for the next step ahead of time. To make the heptane glue, combine a 20 cm length of rolled-up double-sided tape with 20 mL heptane in a 50 mL centrifuge tube, seal with the paraffin film, and rock at room temperature overnight on the bench.
2. Preparation and Dissection of Drosophila Pupae
- Transfer the staged Drosophila pupae to a piece of double-sided sticky tape mounted on a glass slide. Position the pupae so that the ventral side is firmly stuck to the tape and the dorsal side is facing upwards (Figure 1B).
NOTE: The anterior of the pupa will be identifiable from the two spiracles protruding from the anterior end of the pupal case. - Carefully remove pupae from their protective puparium casing under a brightfield dissection microscope using forceps and micro-scissors (Figure 1B-D).
- Initially, make an incision in the anterior-most region of the puparium using the forceps (Figure 1B). Ensure that the pupal case in this area is hollow and devoid of pupal tissue because the pupae will have shrunk within the case during early pupal development.
- After this initial incision, carefully tear or cut the pupal case open in an anterior-to-posterior direction using forceps or microscissors (Figure 1C) until the pupa is completely free from the brown opaque brittle casing (Figure 1D).
NOTE: Pupae at this stage are very fragile and care must be taken to avoid puncturing the pupal surface; puncturing is obvious as hemolymph quickly leaks out from the puncture site. Pupae with punctures, however small, should be discarded.
- Mount the pupae in a glass-bottomed dish using heptane glue.
- Use a 20 mL pipette tip to place a 10 mL drop of pre-prepared heptane glue (see section 1.6 above) in a line on the glass-bottomed dish.
- Allow the glue to dry for 5 s before transferring the dissected pupae carefully onto the heptane glue using forceps (Figure 1E).
- For ease of wounding and imaging, line the pupae up in a row; approximately 5 pupae are suggested but more will be manageable with experience.
NOTE: The use of heptane glue is recommended when using upright imaging systems but is not necessary when using an inverted system. If the glue creates optical aberrations during imaging, pupae can be placed directly on the coverglass instead – the natural adhesion between the pupal tissue and glass will be sufficient in most cases for stable imaging, as long as care is taken when moving the imaging dish between microscopes.
- For best results, mount the pupae so that the wing is flat on the coverglass with the majority of the wing surface in direct contact with the coverglass (Figure 1F). Roll the pupae using forceps to change their position to ensure the wing is mounted correctly.
- To prevent sample dehydration during the imaging period, add a piece of absorbent filter paper soaked in distilled water to the side of the glass-bottomed dish at the end of mounting, being careful not to disturb the pupae (Figure 1E). Cover the dish with a lid.
NOTE: Pupae are now ready for wounding and imaging.
3. Laser-induced Wounding of Drosophila Pupal Wings
- Transfer the glass-bottomed dish containing the mounted pupae to a wide-field microscope equipped with a tunable laser ablation system.
- Use a pulsed-UV air-cooled nitrogen-pumped ablation laser tuned to 435 nm – see Table of Materials for details; the precise wavelength of the light used for illumination is selected by the user via an appropriate dye cell.
- Using brightfield optics, adjust the microscope stage controls to locate the pupal wing of the first pupa to be wounded (Figure 1F).
- For optimal results, use an oil immersion 40X or 63X objective lens for both the imaging and laser ablation; ensure that the immersion liquid used (oil or glycerol) is consistent between ablation and imaging systems. Adjust the microscope to focus on the plane of the pupal wing epithelium nearest the glass coverslip (i.e. focus on the region of the epithelium to be wounded) using the fine focus control knob.
- Using the microscope stage adjustments, position the pupal wing so that the area to be wounded is directly aligned with the known target area of the ablation laser.
- Use an energy density attenuator slide fitted to the microscope to manually adjust the power level of the ablation light.
NOTE: The attenuator slider has click stops and rulings to identify the relative level of attenuation and permit the use of reproducible settings. - Using an external manual trigger control, activate the ablation laser using a single brief click of the trigger to make a wound. Check for the appearance of the transient air bubble at the ablation site since wounding will normally be accompanied by it. Check if the laser-induced wounding has been successful using the appropriate fluorescent filters to visualize the pupal epithelium.
NOTE: Take care to avoid accidental exposure to the laser beams as the beam reflections can cause severe eye or skin damage. - If wounding is unsuccessful, vary the focal plane (moving the microscope focus slightly above or below the current focal level) and repeat the single click of the ablation trigger. Alternatively, gradually increase the laser power using the attenuator slide until the desired wound size is achieved.
- To vary the ablation laser pulse repetition rate, use the Repetition rate knob on the rear control panel (which changes the rate from less than 1 pulse/s up to 60 Hz). For optimal wounding, set the pulse repetition rate to 40 Hz.
- To generate different-sized wounds, use the energy density attenuator slide to manually adjust the power level of the ablation light.
- Refrain from wounding all of the mounted pupae and use these non-ablated pupae as unwounded controls.
- For consistent results, regularly realign the ablation system (using the relevant operating manual). Also, clean and refill the dye resonator cell that controls the laser output wavelength.
4. In Vivo Time-lapse Confocal Imaging
- Quickly transfer the glass-bottomed dish to an appropriate microscope for time-lapse imaging.
NOTE: For optimal results, use a high-specification confocal or spinning disc microscope equipped with sensitive detectors that can detect both green fluorescent protein (GFP) and mCherry fluorophores. - To image the entire pupal wing, use a low magnification (e.g. 20X) objective lens (Figure 2A). To image wound repair and the accompanying inflammatory response with high spatial resolution, use the oil immersion 40X (NA 1.3) or 63X (NA 1.4) objective lenses (see representative images in Figure 2B-D).
- Open the appropriate image capture software associated with the microscope.
- Using the image capture software, turn on the appropriate lasers e.g. the 488 nm and 561 nm lasers to visualize GFP and mCherry fluorophores, respectively (by clicking in the relevant boxes) and adjust the laser power and gain/offset settings to give sufficient fluorescent signal whilst avoiding pixel saturation; use the lowest possible laser power (in the range 5 – 20%) to minimize photobleaching and phototoxicity.
- To capture both the repairing epithelium and inflammatory cell recruitment, set the microscope to record a z-stack using the fine focus adjustment knobs on the control panel; for optimal results, set the software (using manual button-clicks) to record z-slices through the pupal wing (minimum every 3 mm), from the top of the wounded epithelium through to the extracellular space beneath (containing migrating hemocytes) to achieve a large z-stack (in the range of 50 - 100 mm).
- For time-lapse imaging, record z-stacks at regular time intervals (minimum every 30 s) for at least 1 h post-wounding.
NOTE: The exact time interval between the z-stacks chosen represents a trade-off between capturing the rapidly changing cell dynamics and avoiding photo-bleaching of the samples. - To simultaneously image multiple pupae (including non-ablated unwounded controls), use a motorized stage (attached to the microscope) and the multi-position acquisition feature available within the imaging software. Manually set the position of each pupa within the software using the stage position control knobs and then manually set the appropriate z-stack limits (top and bottom) for each pupa.
- Visualize the time-lapse images either during image capture or later with specialist image analysis software (such as ImageJ) using z-stack projections or 3-D rendering. For example, to follow the movements of individual hemocytes (as in Figure 2C' and D'), track hemocyte nuclei using the open-access ImageJ plug-ins "TrackMate" or "Manual Tracking" (methods published in).
- Use photoconvertible probes (such as Kaede) to selectively photoconvert and label a subset of epithelial or immune cells during imaging.
- Open appropriate modules within the imaging software to perform the photoconversion (such as the Fluorescence recovery after photobleaching (FRAP), and fluorescence recovery after photo-bleaching module) and activate the 405nm laser (by clicking in the relevant software box).
- Select cells to be photoconverted within the FRAP software using the square, circular, or freehand selection tool. Within the FRAP software, set the time course for photoconversion (Bleaching) to a single iteration/frame and set the 405 nm laser at 20% laser power. Manually click Start the experiment to perform photoconversion.
- Exit the FRAP module (click Close) and return to the original imaging screen within the software; use the 488 nm and 561 nm lasers to image the behavior of photoconverted and non-photoconverted cells by setting up a z-stack and time-lapse recording as above.
NOTE: Photoconverted probes remain stable for many hours after the initial photoconversion, enabling the behavior of the photoconverted cells to be followed over time (for at least 5 h). For example, inflammatory cells in the wound can be selectively photoconverted (Figure 2F) and their behavior followed as they resolve from the injury site (Figure 2G and H).
Representative Results
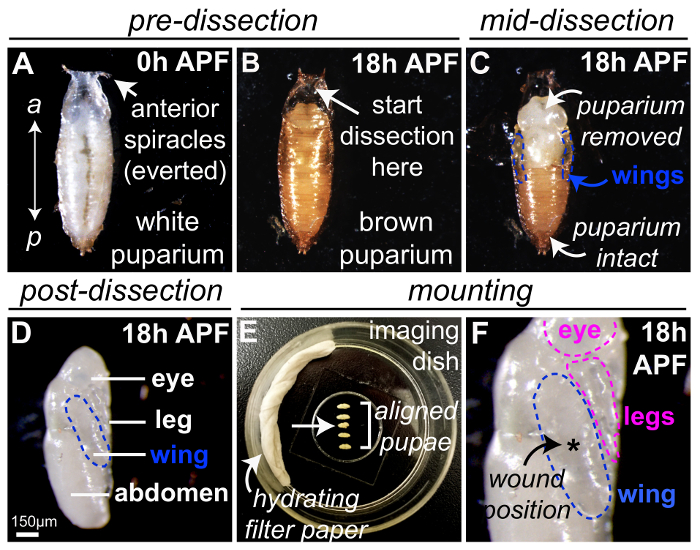
Figure 1: Drosophila pupa preparation for wounding and live-imaging. (A) Drosophila white prepupae collected at 0 h APF, with the anterior end indicated by everted breathing appendages (spiracles). (B) After raising white 0h APF prepupae for 18 h at 25 °C, the puparium appears brown. Dissection of the pupal case should begin at the anterior-most region (arrow), indicated by the everted spiracles as the pupa proper is absent from this region, and fine forceps and/or microscissors used to remove the protective pupal case (C). The pupal wings will be visible on the lateral sides of the pupal thorax (blue outlines). (D) The pupal case was completely removed from the 18h APF pupa, here the pupa has been rolled 90° to show the lateral side, with the wing to be imaged outlined in blue. (E-F) Five 18h APF pupae were mounted on a glass coverslip within the imaging dish using heptane glue, with water-soaked filter paper to minimize dehydration (E). The pupa located third in the sequence (arrow) should be discarded as damage occurred during preparation. Pupae are mounted with the flattest portion of the wing (outlined blue) in contact with the coverslip (F) and laser-induced wounds are generated centrally in the wing (asterisk, F), although other locations may be used if multiple wounds are to be studied. The image in (D) adapted with permission from Weavers et al., 2016.
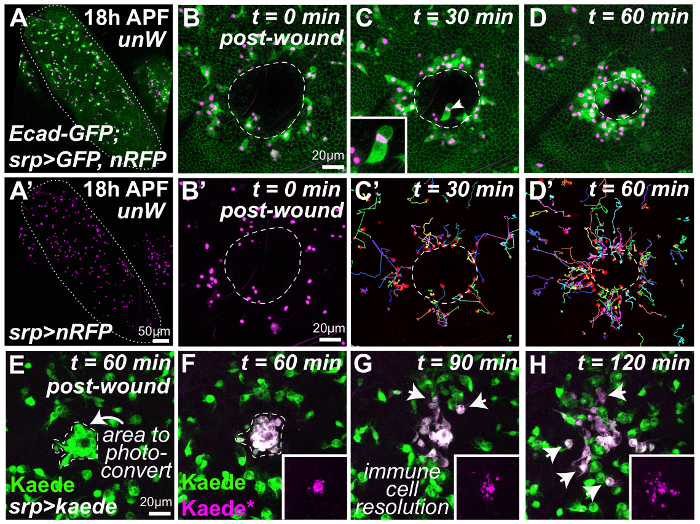
Figure 2: Dynamic in vivo analysis of the inflammatory response to tissue damage. Pupae dissected from protective pupal cases and mounted on glass coverslip (A) are wounded and subsequently imaged using confocal time-lapse microscopy (B-H). Low magnification view of the unwounded pupal wing (A, wing margin outlined in white) that contains large numbers of migratory hemocytes (A'). Laser-induced injury to the pupal wing epithelium (B-D, cell boundaries labeled using GFP-tagged Drosophila E-cadherin; wound margin outlined in white) activates a rapid inflammatory response with the migration of multiple hemocytes (srp-Gal4 driven expression of nuclear RFP, magenta and cytoplasmic GFP, green) towards the wound site (B-D; representative frames from a time-lapse movie in which each frame is a projection of 25 slices 3 μm each). Manual tracking of hemocyte trajectories (multi-colored tracks, C' and D') indicate the complex spatiotemporal dynamics of the inflammatory response, similar to that reported for wounded embryos. Hemocytes also phagocytose necrotic cellular debris at the wound site (arrowhead, C, and inset). Expression of the photoconvertible fluorophore Kaede in the immune cell lineage (green, E, using srp-Gal4) enables wound-recruited hemocytes (arrow, E) to be differentially labeled (magenta, F) and followed over time as they resolve from the injury site (arrows, G and H). The following pupal genotypes were utilized: (A-D) w1118;ubi-DE-cad-GFP, srp-Gal4>UAS-GFP(II); UAS-nRFP(III) and (E-H) w1118;srp-Gal4(II); UAS-Kaede(III). Images adapted with permission from Weavers et al., 2016.
Divulgazioni
The authors have nothing to disclose.
Materials
| Drosophila stocks | |||
| Ubiquitous GFP-tagged E-cadherin;Ubi-p63E-shg.GFP; (chrII) | Kyoto Stock Center, DGRC | #109007 | Ubi-p63E promoter sequences drive the expression of Drosophila E-cadherin (shotgun) tagged at the C-terminal end with GFP. |
| Ubiquitous GFP-tagged E-cadherin;;Ubi-p63E-shg.GFP (III) | Bloomington Drosophila Stock Centre (Indiana University) | #58742 | Ubi-p63E promoter sequences drive the expression of Drosophila E-cadherin (shotgun) tagged at the C-terminal end with GFP. |
| Ubiquitous GFP-tagged Moesin P{sGMCA}3.1 | Bloomington Drosophila Stock Centre (Indiana University) | #59023 | The ubiquitously expressed sqh promoter/enhancer drives expression of a fragment of Moesin (that includes the actin binding sequences) tagged with GFPS65T. |
| Hemocyte specific serpent-Gal4 driver ;srp-Gal4; | Generated by Katja Bruckner | Generated by Katja Bruckner | The expression of ScerGAL4 fused to a polyA tail is controlled by 2 genomic sequences from upstream of Drosophila serpent. Ref: Brückner, K., Kockel, L., Duchek, P., Luque, C.M., Rørth, P., Perrimon, N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 7 (1), 73–84, doi: 10.1016/j.devcel.2004.06.007 (2004). |
| UAS-nuclearRFP w1118;;P{UAS-RedStinger}6 | Bloomington Drosophila Stock Centre (Indiana University) | #8545 or #8547 | UAS regulatory sequences drive expression of the DsRed.T4 form of RFP which is tagged at the C-terminal end with a nuclear localisation signal |
| UAS-cytoplasmicGFP ;;P{UAS-GFP.S65T} | Bloomington Drosophila Stock Centre (Indiana University) | Multiple stocks available (e.g. #1522) | Expression of the S65T version of GFP by UAS regulatory sequences; the S65T variant exhibits increased brightness. |
| UAS-photoconvertibleKaede w1118;; P{UAS-Kaede.A}3 | Bloomington Drosophila Stock Centre (Indiana University) | #26161 | Kaede protein emits bright green fluorescence after synthesis, but changes efficiently to a bright stable red fluorescence on irradiation with UV. |
| GFP-tagged spaghetti squash w1118;;P{sqh-GFP.RLC} | Bloomington Drosophila Stock Centre (Indiana University) | #57145 | The sqh coding region, which is tagged at the C-terminal end with a T:AvicGFPS65T tag, is expressed under the control of the natural sqh promoter. |
| Ingredients for fly food media | Fly food media is made according to standard procedures (see Greenspan, R. 1997. Fly Pushing: The Theory and Practice of Drosophila Genetics. Cold Spring Harbor Press. 1-191 pp.) | ||
| Maize | Wild Oats, Bristol, UK (or equivalent supplier) | Contact supplier direct | organic |
| Soya flour | Wild Oats, Bristol, UK (or equivalent supplier) | Contact supplier direct | organic |
| Malt extract | Wild Oats, Bristol, UK (or equivalent supplier) | Contact supplier direct | organic |
| Molasses | Wild Oats, Bristol, UK (or equivalent supplier) | Contact supplier direct | organic |
| Difco agar | BD Biosciences, Fisher Scientific | DF0142-15-2 | For preparation of fly food |
| Propionic acid | Sigma | 402907 | For preparation of fly food |
| Nipagen | Sigma | 79721 | For preparation of fly food |
| Dried baker's yeast | Redstar, Dutscher Scientific, UK LTD | Redstar, Dutscher Scientific, UK LTD | For preparation of fly food |
| Sample preparation and mounting | |||
| Parafilm | Sigma | P7793-1EA | For preparation of heptane glue |
| Fine sable paintbrush | Daler-Rowney (or equivalent) | #0 or 1 | |
| Forceps | Fisher Scientific (or Fine Science Tools) | NC9404145 | Dumont #5 |
| Glass bottomed dishes for imaging | MatTek | P35G-0-10-C | We suggest using 35mm petri dishes, with at least a 10mm Microwell, 0.085-0.13mm cover glass, uncoated. Dishes with larger microwells will enable increasing numbers of pupae to be mounted and imaged in a single experiment. |
| Heptane | Sigma | 51730-5ML | For preparation of heptane glue |
| Double sided sticky tape (e.g. Scotch) | Agar Scientific | AGG263 | For preparation of heptane glue |
| 50ml tube (for heptane glue) | Falcon tubes from Fisher Scientific | 14-432-22 | For preparation of heptane glue |
| Glass microscope slides | Agar Scientific | AGL4244 | For dissection of Drosophila pupae |
| Dissecting stereo microscope with brightfield | Leica (or equivalent) | M50 | For dissection of Drosophila pupae |
| Microscissors | John Weiss International | 103123 | Miniature Research Scissors (straight) |
| Laser ablation and imaging | |||
| Nitogen ablation laser | Spectra-Physics (or Andor equivalent) | Model VSL-337ND-S | For wounding, this should be attached to a widefield imaging system |
| Multilaser confocal laser-scanning microscope (CLSM) | Leica (or equivalent) | TCS AOBS SP8 or SP5-II attached to a Leica DMi8 inverted epifluorescence microscope (or equivalent) | Ideally including a motorised stage for multi-site and 'mosaic' scanning, plus 'hybrid' GaAsP detectors (that offer much greater sensitivity and boosting of low signal) |
| Environmental chamber | Life Imaging Services (or equivalent) | "Microscope Temperature Control System" | Attached to Confocal microscope for temperature control during imaging |
| Image Analysis Software | |||
| FRAP software module | Leica (or equivalent) | CLSM FRAP software module | For performing photoconversion of photoconvertible fluorophores such as Kaede |
| ImageJ (image analysis software) | National Institutes of Health (NIH) | https://imagej.nih.gov/ij/ | Schneider, C.A., Rasband, W.S., Eliceiri, K.W. "NIH Image to ImageJ: 25 years of image analysis". Nature Methods 9, 671-675, 2012. |
| ImageJ plugin "Manual Tracking" | National Institutes of Health (NIH) | https://imagej.net/Manual_Tracking | |
| ImageJ plugin "TrackMate" | ImageJ, NIH | https://imagej.net/TrackMate | Tinevez, JY.; Perry, N. & Schindelin, J. et al. (2016), "TrackMate: An open and extensible platform for single-particle tracking.", Methods 115: 80-90, PMID 27713081 |
| Volocity (high performance 3D imaging software) | Perkin Elmer | Volocity 6.3 | For image analysis |
| IMARIS (image analysis software) | Bitplane | IMARIS for Cell Biologists | For image analysis |

