An Immunological Technique to Monitor Adjuvant-Mediated Cytotoxic T Lymphocyte Generation
Abstract
Source: Lirussi, D. et al., Rapid In Vivo Assessment of Adjuvant's Cytotoxic T Lymphocytes Generation Capabilities for Vaccine Development. J. Vis. Exp. (2018)
The video demonstrates an immunological method to monitor in vivo adjuvant-induced cytotoxic T lymphocyte or CTL production. A mouse is pre-injected with genetically engineered, proliferative dye-stained donor CD8+ T lymphocytes. Subcutaneous injection of ovalbumin with adjuvants generates cytotoxic CD8+ T lymphocytes that are identified post-harvesting through immunostaining and flow cytometry.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
All mice used in this study were from the C57BL/6 background. All the animals were kept under pathogen-free conditions.
1. Carboxyfluorescein succinimidyl ester (CFSE) Staining of OT-I T Cells and Adoptive Transfer
NOTE: OT-I mice are transgenically generated animals that express a T cell receptor (TCR) with fixed α and β chains that together recognize the immuno-dominant peptide of ovalbumin, OVA, SIINFEKL. As a result, these mice have a considerably high number of SIINFEKL-specific CD8+ T cells (97%) when compared to normal or OVA-vaccinated mice (≤ 1%).
- Isolation of traceable CD8+ T cells from OT-I mice expressing T lymphocyte-specific Thy1a (Thy1.1) allele:
- Euthanize 6-9 weeks old OT-I mice by carbon dioxide (CO2) inhalation followed by cervical dislocation. Dissect the spleen and major lymph nodes (inguinal, axillary, and cervical pairs only) by cutting the skin with surgical scissors, detaching the skin from the body with the help of surgical pliers and forceps, and place them in Petri dishes (60 x 15 mm) each containing a 100 µm pore mesh cup for tissue grinding and cell release.
- Maintain Petri dishes (each with one mesh cup) in 3-5 mL of complete Roswell Park Memorial Institute, RPMI medium (RPMI 1640, 10% v/v fetal calf serum (FCS), 100 U/mL penicillin, 50 µg/mL streptomycin) kept on ice.
- Mash the organs with the help of a syringe plunger or a similar sterile instrument (prior cutting is not needed) and collect the resulting single-cell suspension in 15 mL centrifuge tubes.
- Centrifuge the cell suspension at 300 x g for 10 min at 4 °C. Discard the supernatant. Wash the cells in 10 mL of cold phosphate-buffered saline (PBS) by centrifugation and lyse the erythrocytes from the spleen by re-suspending the pellet in 1 mL/spleen of ammonium chloride buffer (ACK buffer, commercially available). Incubate the cells for 1.5 min on ice and subsequently wash the cells with 10 mL of cold PBS by centrifugation (as done before).
- Re-suspend the pellet in the same tube in 1 mL of PBS (pH 7.2) containing 5% fetal bovine serum for magnetic isolation.
- To perform magnetic isolation, proceed to the negative selection of CD8+ T cells by using a magnetic isolation kit according to the manufacturer's instructions or published protocols.
- To perform CFSE staining, first count the number of CD8+ T cells obtained from OT-I mice by using an automated cell counter (particle counter). Stain 1 – 5 x 107 cells/mL in a volume of 1-5 mL with 5 µM CFSE in PBS for 7 min at 37 °C, protected from light in a 15-ml falcon tube.
- Quench the CFSE staining by adding the same volume (1:1) of fetal bovine serum to the cells and incubate them for an additional 7 min at 37 °C protected from light. Wash the cells with 10 mL of PBS twice.
- Count the cells using an automated cell counter. Set the cell number to 3-5 x 107 cells/mL of PBS in order to inject 3-5 x 106 cells/mouse in 100 µL intravenously (i.v.) via the tail vein.
- To perform tail vein injections, immobilize the mice in appropriate restrainers. Warm the back area and tail of the mice to be injected by using a red-light lamp, placed between 20 to 25 cm away from the mice for 1-3 min to allow tail vein vasodilation.
NOTE: This helps to ease vein detection and administration of the cell suspension. - Ensure (with the hand placed in between the mice and the lamp) that it is not too hot for the mice and when the tail veins are clearly visible, proceed to injection. Inject the cells into the lateral or dorsal tail vein using a 1mL syringe with a 25-gauge needle.
2. Immunization (Endo-free OVA +/- Adjuvant)
- Place the mice transplanted with OT-I cells (step 1.7, Figure 1) in an anesthesia chamber and administer isoflurane in oxygen by an anesthesia machine.
- When the mouse is completely asleep under anesthesia, take it out from the chamber and shave the fur of the mouse on the area over the gluteus superficialis (lateral lower back) by using an electric hair trimming machine, in order to perform a clean injection with a good view of the application area.
- Inject 50 µL of the vaccine, s.c. in the shaved area using a 25-gauge needle.
3. Isolation of Lymphocytes and Staining for Flow Cytometry Analysis
- Isolation of lymphocytes and splenocytes
- Euthanize the vaccinated mice by CO2 inhalation followed by cervical dislocation.
- Extract the draining and distant lymph nodes and spleen and place them in separate Petri dishes containing 100 µm pore mesh cups for tissue grinding and cell release. Follow steps 1.1.2 through 1.1.3.
- Decant the supernatant after centrifugation and re-suspend the cell pellets in the same volume of remnant PBS (approx. 100 µL).
- Staining for flow cytometry analysis
- In a 15-mL centrifuge tube prepare a master mix containing the staining antibodies in the concentrations depicted in Table 1, thus yielding a 2x concentrated staining mix. Prepare enough volume of master mix to have 100 µL per sample. Mix the cells and the master mix 1:1 and incubate it at 4°C for 30 min.
NOTE: The final staining volume per sample should be 200 µL (100 µL of cell pellet +100 µL of antibody master mix). - Wash the cells twice by centrifugation as described in 1.1.3, adding 10 mL of PBS, twice.
- Re-suspend the stained cells in 0.5-1 mL of PBS for acquisition and transfer the suspension to flow cytometer tubes. Always keep the samples on ice and protected from light.
- In a 15-mL centrifuge tube prepare a master mix containing the staining antibodies in the concentrations depicted in Table 1, thus yielding a 2x concentrated staining mix. Prepare enough volume of master mix to have 100 µL per sample. Mix the cells and the master mix 1:1 and incubate it at 4°C for 30 min.
4. Flow Cytometry
- Always pre-filter the cell samples using 70-100 µm filters.
- Prepare single staining compensation controls for the fluorophores detailed in Table 1 (beads or cells).
NOTE: Compensation should be performed in cytometer or analysis software and applied to all samples. - Follow the gating strategy depicted in Figure 2. Briefly, gate populations in forward scatter height vs. forward scatter area (Figure 2A), and again side scatter wide vs. side scatter area (SSA, Figure 2B) in order to exclude doublets.
- Gate the population from Figure 2B by plotting BV 650 (auto-fluorescence) vs. fluorescein isothiocyanate, FITC (CFSE) in order to discriminate true CFSE stained cells from high auto-fluorescent cells. Include beads or cells stained with antibody conjugated to BV 650 into compensation controls. This plot (Figure 2C) of BV 650 vs. FITC is used to gate the OT-I CFSE stained cells.
- Gate the population from Figure 2C for Pe-Cy7 (Thy1.1 -CD90.1-) vs. allophycocyanin, APC (CD8) in order to distinguish cells derived from donor vs. recipient mice.
NOTE: Cells derived from OT-I donor mice are Thy1.1+ (CD90.1), whereas WT (C57BL/6, recipient) mice-derived cells are Thy1.2+ (CD90.2) (Figure 2D). Some laboratories have OT-I mice in a Thy1.2 background and WT (C57BL/6) in a Thy1.1. In this case, you have to use an antibody against Thy1.2 for OT-I cell gating. - Re-gate CD8+ cells from Thy1.1+ population by plotting it on a gate comprising BV 450 (CD4) vs. APC (CD8) (Figure 2E).
- Display the population gated in Figure 2E by using a histogram display of the CD8+ cells showing CFSE (Fluorescein isothiocyanate, FITC, 530/15 channel -blue laser-), gate the proliferated population by including intensities from 102 up to the level where the undivided control populations are (intensities ~105, depending on the efficacy of the CFSE staining and the voltage setting on the flow cytometer) (Figure 2F).
- Make an additional gate (not displayed in Figure 2) plotting FITC vs. L/D marker (450/20 channel, ultraviolet (UV) laser) in order to assess dead cells in parallel to the proliferation evaluation.
- Acquire at least 5000 events for the compensation controls and 10.000 events (gate E, Figure 2) from the samples at a flow cytometer. Run the cytometer at low or medium flow (do not exceed flow rates of 20.000 events/s).
Table 1: Antibody stainings for flow cytometry. Fluorophore-conjugated antibody clones used and recommended staining concentrations (2x).
| Dyes – Antibodies | Clone | Fluorophore/channel-filter | Concentration 2X |
| Thy1.1 (CD90.1) | HIS51 | PE-Cy7 – 780/60 YG | 1:750 |
| CD8 | 53-6.7 | APC – 670/14 R | 1:280 |
| CD4 | RM4-5 | BV 421 – 450/50 V | 1:100 |
| CFSE | – | FITC – 530/30 YG | (according to CFSE-staining protocol) |
| DCM (Dead Cell Marker) | – | -/ U.V. – 450/50 UV | 1:500 |
Representative Results
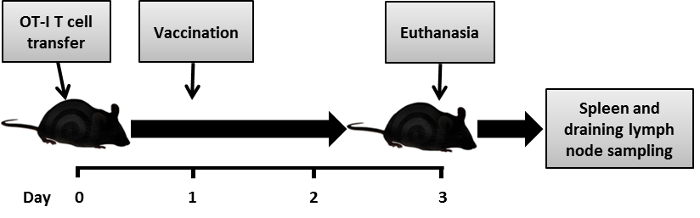
Figure 1: The assay timeline. The assay timeline represents the initial OT-I T cell transfer at day 0, the s.c. vaccination at day 1, and the sampling spleen and draining of lymph nodes 2 days later.
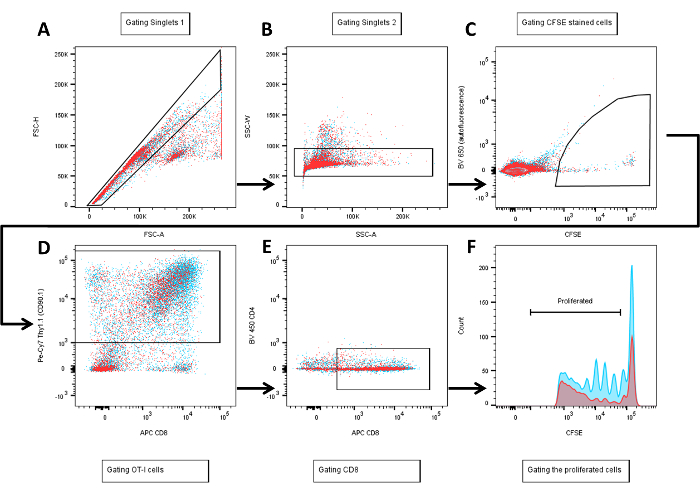
Figure 2: Flow diagram of the gating strategy followed to measure the proliferation of CD8+ T cells (OT-I, Thy1.1+) by flow cytometry. Two samples are represented in different colors (red and light blue) for better visualization. A-B. Single cells are discriminated from doublets successively in the first two gates by plotting forward-scatter-height vs. forward-scatter-area and side-scatter-width vs. side-scatter-area. C. Cells gated in B are displayed by their fluorescence intensities of the BV 650 channel (autofluorescence) plotted against their CFSE intensity (where diming indicates cells' divisions/proliferation) in the 530/30 YG channel. This gate allows the selection of true CFSE-positive cells by discriminating those that have high autofluorescence. D. CFSE positive cells were gated with their high fluorescence intensity of Pe-Cy7 (Thy1.1, marker for OT-I cells) vs. their APC intensity (CD8). E. BV 450 (CD4) plotted against APC (CD8) for previously gated Thy1.1 positive cells to accurately select CD8+ T cells. F. Previously gated CD8+ cells are plotted in a histogram against the CFSE intensity to finally gate the proliferated population.
Divulgazioni
The authors have nothing to disclose.
Materials
| BD LSR Fortessa Cell Analyzer | BD | Special Order | Flow Cytometer |
| CFSE | Molecular Probes | C34554 | Proliferation Dye |
| MojoSort Mouse CD8 T Cell Isolation Kit | Biolegend | 480007 | Magnetic Isolation Beads and antibodies for negative selection of untouched CD8 T cells. |
| LIVE/DEAD Fixable Blue Dead Cell Stain Kit, for UV excitation | Molecular Probes | L23105 | Dead Cell Marker |
| CD90.1 (Thy-1.1) Monoclonal Antibody (HIS51), PE-Cyanine7 | eBioscience | 25-0900-82 | antibody |
| APC anti-mouse CD8a Antibody | BioLegend | 100712 | antibody |
| BV421 Rat Anti-Mouse CD4 | BD | 740007 | antibody |
| Z2 coulter Particle count and Size Analyzer | Beckman Coulter | 9914591DA | Cell counter. Z2 Automated particle/cell counter |
| EndoGrade Ovalbumin (10 mg) | Hyglos(Germany) | 321000 | Ovalbumin endotoxin free tested. |
| Cell Strainer 100µm nylon | Corning | 352360 | Cell strainer (100 µm pore mesh cups). |
| Sample Vials | Beckman Coulter | 899366014 | Sample vials for Z2 automated counter |
| C57BL/6 mice (CD90.2) | Harlan (Rossdorf, Germany) | Company is now Envigo | |
| OT-I (C57BL/6 background, CD90.1) | Harlan (Rossdorf, Germany) | Inbreed at our animal facility. Company from where adquired is now Envigo | |
| FACS tubes | Fischer (Corning) | 14-959-5 | Corning Falcon Round-Bottom Polystyrene Tubes |
| Falcon 15 mL tubes | Fischer (Corning) | 05-527-90 | Falcon 15mL Conical Centrifuge Tubes |
| PBS (500 mL) | Fischer (Gibco) | 20-012-027 | Gibco PBS (Phosphate Buffered Saline), pH 7.2 |
| Red lamp (heating lamp) | Dirk Rossmann GmbH (Germany) | 405096 | Heating infrred lamp (100 wats) |
| IsoFlo (Isoflurane) | Abbott Laboratories (USA) | 5260.04-05. | Isoflurane anesthesic (250 mL flask). |
| Tabletop Anesthesia Machine/Mobile Anesthesia Machine with CO2 Absorber | Parkland Scientific | V3000PK | Isoflurane anesthesia machine. |
| RPMI 1640 medium | Gibco (distributed by ThermoFischer) | 11-875-093 | Base medium with Glutamine (500 mL) |
| Pen-Strept antibiotic solution (Gibco) | Gibco (distributed by ThermoFischer) | 15-140-148 | Gibco Penicillin-Streptomycin (10,000 U/mL) |
| Fetal Bobine Serum (Gibco) | Gibco (distributed by ThermoFischer) | 10082147 | Fetal Bovine Serum, certified, heat inactivated, US origin |
| ACK Lysing Buffer (100 ml) | Gibco (distributed by ThermoFischer) | A1049201 | Amonium Chloride Potasium (ACK) Whole Blood Lysis Buffer, suitable for erytrocyte lysis in spleen suspensions also |
| Plastic Petri Dishes | Nunc (distributed by ThermoFischer) | 150340 | 60 x 15mm Plastic Petri Dish, Non-treated |
| Cell Clump Filter | CellTrics (Sysmex) | 04-004-2317 | CellTrics® 50 μm, sterile |

