Targeting Liver Tumors with Oncolytic Viruses via the Hepatic Artery
Abstract
Source: Altomonte, J. et al., Transarterial Administration of Oncolytic Viruses for Locoregional Therapy of Orthotopic HCC in Rats. J. Vis. Exp. (2016)
The video demonstrates locoregional delivery of oncolytic viruses through hepatic arterial injection as a therapeutic approach for hepatocellular carcinoma. These viruses selectively replicate in tumor cells, inducing cell lysis and releasing tumor cell-associated antigens. This process triggers immunogenic cell death, inducing an anti-tumor response.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparations before beginning surgery
- Sterilize surgical instruments (i.e., by autoclaving).
- Dilute the virus to the appropriate concentration, which will vary depending on the toxicity of the specific virus being used and the susceptibility of the rat strain.
NOTE: For example, when using vesicular stomatitis virus in male Buffalo rats, we typically inject 107 plaque-forming unit (pfu) in a 1 ml volume, which is the maximum tolerated dose in that model.- Prefill the virus (using aseptic technique) in 1 ml syringes, and attach 30 G needles, taking care to eliminate air from the syringe and needle.
- Prepare the surgical space with all materials and equipment required, and spray the area with ethanol to disinfect. Fill a small receptacle with sterile saline solution (0.9% sodium chloride, NaCl) for moistening gauze swabs and cotton-tipped applicators.
2. Preparation of the Rat
- Administer an analgesic (as specified by the local animal care and use committee), at the appropriate dose 30 min prior to beginning the surgical procedure. For example, administer a high dose of metamizole (100-200 mg/kg).
- Anesthetize the rat using inhalation (isoflurane) or injectable (i.e., a mixture of Medetomidine (0.15 mg/kg), Midazolam (2 mg/kg), and Fentanyl (0.005 mg/kg), also known as MMF) anesthesia. Administer isolflurane through a vaporizer at 1-3% and adjust as necessary to maintain normal respiration and heart rate.
- Before proceeding, ensure that the rat is fully sedated and unable to feel pain by pinching the foot pad and observing for a reaction.
- Apply a veterinary eye ointment to prevent dryness during anesthesia.
- Shave the abdominal area from the sternum down to just above the genitals using a small veterinary hair clipper. Take care to achieve a close shave without injuring the skin.
- Fix the rat to the surgical bed using tape on the fore- and hindlimbs, and, if using inhalation anesthesia, place the snout into a suitably sized nose cone. Place a heating pad underneath the rat to maintain body temperature. Disinfect the abdominal area using a surgical grade disinfectant, followed by an alcohol rinse and application of an antiseptic paint.
3. Laparotomy
- Using a small, curved scalpel, such as #15, make a small vertical incision in the skin layer directly along the midline. Extend the incision up to the xyphoid process. Next, incise the muscle layer by gently lifting up with a pair of Adson forceps to make a scalpel incision, and then extend the incision up to the xyphoid process with surgical scissors. Take care not to puncture the organs of the abdominal cavity or to damage the small vessels on either side of the xyphoid process.
- Place a retractor across the abdomen to spread the muscle and skin and expose the entire abdominal area.
- Moisten two 7.5 x 7.5 cm2 gauze swabs with sterile physiological saline and spread one across the top of the surgical opening and one across the bottom, as shown in Figure 1A.
4. Isolation of the Hepatic Artery
- Using two moistened cotton applicator swabs moistened with sterile physiological saline, gently lift the intestines and cecum out of the abdominal cavity, and place them on the lower gauze swab. Fold the gauze over to keep the organs moist and to hold them out of the way.
- Using two moistened cotton applicator swabs, gently lift up the left lateral liver lobe, which will be the most predominant lobe in view at this stage. Flip the lobe upwards, and, using a small pair of spring scissors, cut through the fibrous membrane on the underside of the lobe, allowing the lobe to be completely flipped upwards and rest on top of the previously placed gauze swab. Fold the gauze swab over the lobe to hold it in place.
- Continue with the next lobe (the anterior caudate), which is now the most anterior lobe in view. With the aid of a dissecting microscope and fine forceps, dissect the thin membrane surrounding the lobe in order to release it and allow it to be freely flipped upwards. Place the lobe underneath the gauze swab together with the left lateral lobe.
- Observe the posterior caudate lobe, which will now be visible. Repeat step 4.3 to completely dissect the posterior caudate lobe and flip it upwards and inside the gauze together with the previously placed liver lobes.
- Just beneath the original position of the caudate lobe, find the common hepatic artery, which will now be visible and easily recognized by its strong pulsation. Following the artery to the anatomical right, observe the thick membranous material obscuring the gastroduodenal and proper hepatic arteries from view. Using fine forceps, lift up this material and gently tear it, taking care to avoid any small vessels in this area. Use cotton applicator swabs to stop any bleeding which might occur.
- Locate the arterial branches, which will be clearly visible with the aid of a dissecting microscope when the membranes are properly dissected.
- Using two fine, atraumatic forceps, carefully dissect the common hepatic artery, gastroduodenal artery, and the proper hepatic artery such that they are freed from the surrounding membranes (Figure 1B).
5. Preparation of the Artery for Injection
- Pass a 7-0 Prolene suture underneath the gastroduodenal artery, and use a micro-needle-holder to tie a permanent ligature at the distal end, just above the bifurcation (see Figure 2 for proper placement). Allow approximately 2 inches of suture material remaining on the ends, and clamp them with a needle holder to pull the artery taught, as in Figure 1C.
- Place a small clamp on the common hepatic artery to temporarily block blood flow (Figure 1C).
NOTE: This is important to prevent massive bleeding once the needle is removed from the artery. Furthermore, the blockage of arterial blood flow will allow for slower virus administration and better transduction efficiency.- Important: Work quickly to limit the amount of time that blood flow is stopped – perform the injection and subsequent ligation of the artery within 5 min of clamping the common hepatic artery.
- If possible, increase the magnification on the dissecting microscope, such that the gastroduodenal artery is in sharp focus and appears large enough to accurately insert the 30 G needle. Place the needle of the injection syringe into the lumen of the gastroduodenal artery and slowly push on the plunger.
NOTE: Proper placement of the needle will result in the injected solution flowing directly through the proper hepatic artery and towards the liver.- Perform the injection very slowly to maximize transduction efficiency.
- Once the entire injection volume has been administered, slowly retract the needle from the artery.
NOTE: There should be no bleeding from the injection site. In the case that bleeding does occur, it indicates that the clamp is not properly placed and should be quickly adjusted.
6. Closing
- Pass a second 7-0 Prolene suture beneath the gastroduodenal artery, and tie a second permanent ligature above the injection site.
- Remove the clamp from the common hepatic artery. Confirm that blood flow through the proper hepatic artery towards the liver has been restored.
- Cut the loose ends of the ligatures.
- Confirm that there is no bleeding before returning the intestines and liver to the abdominal cavity. Take care that the appendix is returned in its original orientation.
- Suture the muscle and skin in separate layers using 4-0 vicryl suture material with a curved needle. Place continuous sutures approximately 1 mm apart and pull tightly.
- During the suturing phase, administer an analgesic such as buprenorphine (0.05 mg/kg).
- Remove rat from anesthesia. If isoflurane has been used, the rat should regain consciousness within several minutes. If MMF has been used as an injectable anesthesia, antagonize it with a subcutaneous injection of Atipamezole (0.75 mg/kg), Flumazenil (0.2 mg/kg), and Naloxone (9.12 mg/kg), which will reverse the effects of the anesthesia and allow the animals to regain consciousness within 20 min. Keep rats warm with an infrared warming lamp during the initial wakening phase.
- Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency.
- Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
Representative Results
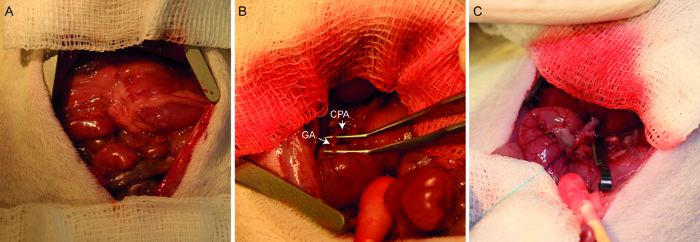
Figure 1. Photographs of the hepatic arterial infusion procedure. A laparotomized rat demonstrates the placement of the abdominal wall retractor and gauze swabs (A). After lifting out the hepatic lobes, the common hepatic artery (designated CHA) and gastroduodenal artery (designated GA) are identified and dissected (B). After ligating the distal end of the gastroduodenal artery, the common hepatic artery is temporarily clamped (C). The gastroduodenal artery is now ready for injection.
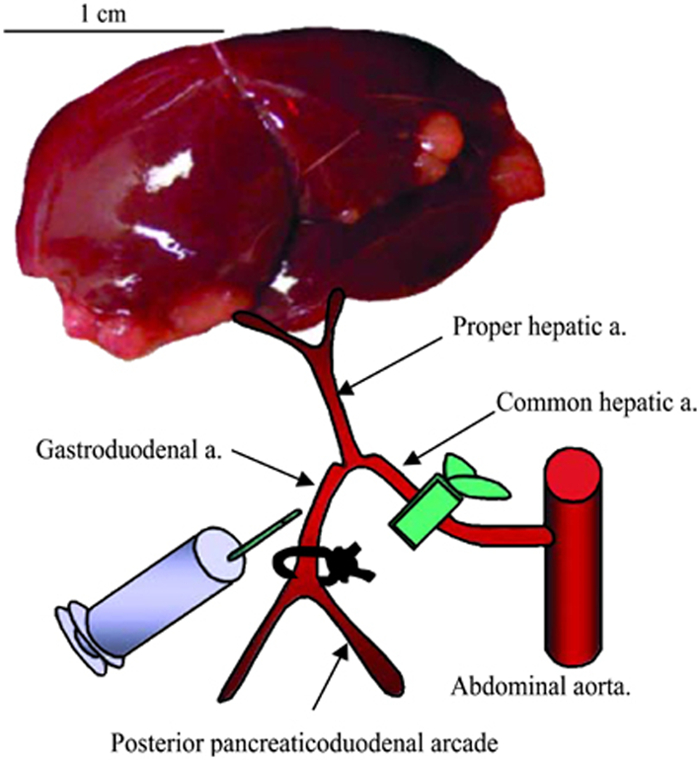
Figure 2. Schematic representation of hepatic arterial infusion procedure. The hepatic vessels (common hepatic artery, proper hepatic artery, and gastroduodenal artery) are dissected with the aid of a dissecting microscope. After ligation of the gastroduodenal artery and temporal block of the common hepatic artery, 1 ml of PBS or VSV vector are administered via the gastroduodenal artery through the proper hepatic artery. After injection, the proximal site of the gastroduodenal artery is ligated to prevent bleeding, the block of the common hepatic artery is released, and the recommencement of hepatic blood flow is confirmed.
Divulgazioni
The authors have nothing to disclose.
Materials
| Veterinary clippers | Aesculap | GT415 | Small, cordless trimmer ideal for removing fur from surgical area |
| Stereomicroscope | Zeiss | Stemi SV6 | |
| 30G Needles | Braun | 4656300 | 30G x ½" |
| 1ml syringes | Braun | 9161406V | Tuberculin syringe |
| Disposable scalpel | Feather | 2975#15 | #15 blade |
| Standard surgical scissors | Fine Science Tools | 14001-13 | Sharp/blunt, for opening skin and muscle |
| Adson forcep | Fine Science Tools | 1101-12 | With teeth, for grasping skin and muscle |
| Alm retractor | Fine Science Tools | 17008-07 | With blunt teeth, for spreading abdominal cavity open during surgery |
| Gauze swabs | Lohmann & Rauscher | 18504 | 7.5 x 7.5 cm, should be autoclaved prior to use |
| Cotton-tipped applicator swabs | Lohmann & Rauscher | 11970 | Sterile |
| Fine-tipped foreceps | Fine Science Tools | 11063-07 | 0.4mm, angled tip, for dissecting hepatic artery |
| Vannas spring scissors | Fine Science Tools | 91500-09 | For delicate cutting |
| Micro-needle holder | Fine Science Tools | 12076-12 | For ligating gastroduodenal artery |
| Needle holder | Fine Science Tools | 12005-15 | Tungsten carbide jaws |
| 7-0 Prolene sutures | Ethicon | 8648H | Polypropylene suture with curved needle, for ligating gastroduodenal artery |
| 4-0 Vicryl sutures | Ethicon | V3040H | With curved needle attached |
| Infrared warming lamp | Beurer | IL11 | For maintaining body temperature post-operatively |
Tags

.
