Measuring Caenorhabditis elegans Life Span in 96 Well Microtiter Plates
Summary
In this protocol we present a method to measure Caenorhabditis elegans lifespan in 96 well microtiter plates.
Abstract
Lifespan is a biological process regulated by several genetic pathways. One strategy to investigate the biology of aging is to study animals that harbor mutations in components of age-regulatory pathways. If these mutations perturb the function of the age-regulatory pathway and therefore alter the lifespan of the entire organism, they provide important mechanistic insights1-3.
Another strategy to investigate the regulation of lifespan is to use small molecules to perturb age-regulatory pathways. To date, a number of molecules are known to extend lifespan in various model organisms and are used as tools to study the biology of aging4-16. The number of molecules identified thus far is small compared to the genetic “toolset” that is available to study the biology of aging.
Caenorhabditis elegans is one of the principle models used to study aging because of its excellent genetics and short lifespan of three weeks. More recently, C.elegans has emerged as a model organism for phenotype based drug screens5,7,16-20 because of its small size and its ability to grow in microtiter plates.
Here we present an assay to measure C.elegans lifespan in 96 well microtiter plates. The assay was developed and successfully used to screen large libraries for molecules that extend C.elegans lifespan7. The reliability of the assay was evaluated in multiple tests: first, by measuring the lifespan of wild type animals grown at different temperatures; second, by measuring the lifespan of mutants with altered lifespans; third, by measuring changes in lifespan in response to different concentrations of the antidepressant Mirtazepine. Mirtazepine has previously been shown to extend lifespan in C.elegans7. The results of these tests show that the assay is able to replicate previous findings from other assays and is quantitative. The microtiter format also makes this lifespan assay compatible with automated liquid handling systems and allows integration into automated platforms.
Protocol
Overview: The protocol is split into four parts. Part 1 describes how to prepare the feeding bacteria. Part 2 describes how to prepare the worm culture. Part 3 describes how to score lifespan. Part 4 shows some representative results, and Part 5 describes how to prepare the required solutions. Lifespan experiments take several weeks to complete. For each step of the protocol week, day, and time of the day is included to facilitate planning. The L4 stage (Day 0) is used as the reference point for the entire protocol.
1. Preparation of Feeding Bacteria
This section describes the preparation of the feeding bacteria. The specific E. coli strain used to feed C.elegans is called OP50. Prior to this protocol, to prevent cross contamination of the worm culture with other bacteria, the OP50 strain has been made Carbenicillin/Ampicillin resistant21. Prepare the OP50 four to five days in advance. All materials coming in contact with OP50 must be sterile.
Day -7: Thursday (week 1): Inoculate 5 mL of TB containing 100 μg/ mL Ampicillin and 0.1 μg/ mL Amphotericin B with a single OP50 colony and incubate over night at 37°C in a bacterial shaker.
Day -6: Friday (week 1).
Morning 8:30. Inoculate early to allow enough time for the culture to reach saturation
- Dilute the overnight culture of OP50 1:2000 in 300 mL TB containing 50 μg/ mL Ampicillin. Incubate the culture in a bacterial shaker for 8-12 hours at 37°C until saturation is reached. Do not allow the culture to grow longer than 14 hours.
- Transfer the OP50 into a sterile, pre-weighted centrifugation tube. Pellet the OP50 by centrifugation for 10 min at 3500 rpm (2200 x g) in a table top centrifuge.
- Discard the supernatant and re-suspend the OP50 pellet in sterile water and re-pellet by centrifugation. Repeat this wash twice.
- After the second wash, carefully remove all the remaining water. No water should be left in the tube. Weigh the centrifugation tube containing the pellet and subtract the weight of the empty centrifugation tube in order to determine the weight of the pellet.
- Thoroughly re-suspend the pellet in S-complete to a concentration of 100 mg/ mL. No clumps should be left.
- The concentration of 100 mg/ mL OP50 should correspond to 2 x1010 bacteria/ mL. Use a photo spectrometer to determine the number of bacteria per mL if the relationship between the optical density and number of bacteria per mL is known. If necessary, adjust the concentration of the OP50 feeding solution to 2 x1010 bacteria/ mL.
- Store the OP50solution at 4°C until it is used for the worm culture.
2. Preparation of a Synchronous Worm Culture
This section describes the preparation of the worm culture. Its goal is to generate an age-synchronous population of worms. All materials coming in contact with C.elegans after the bleach treatment in step 5 must be sterile. Plates are kept at 20°C unless otherwise indicated.
Day -6: Friday (week 1), 4:00 p.m.: Transfer the animals to a fresh plate
- Take a 5-10 day old NGM plate on which the majority of the worm population consists of starved L1 larvae.
- Sterilize a metal spatula by shortly heating it over a Bunsen burner. Let it cool. Use the cooled spatula to cut out agar chunks from the plate containing the starved worms. Transfer several of these chunks onto a fresh 10 cm NGM plate seeded with OP50. Incubate for approx. 65 hours at 20 °C until the majority of the worm population consists of gravid adults. The time it takes for the starved L1 animals to grow into gravid adults may vary from strain to strain.
Day -3: Monday (week 2) 10:00 a.m.: Establish a synchronous population
- Collect worms from the 10 cm plate by washing them off the plate with 5-10 mL sterile water. Transfer the worm/water solution into a 15 mL conical tube.
- Wash the worms by centrifuging 2 min in a tabletop centrifuge at 1200 rpm (280 x g). Discard supernatant and add 10 mL water. Repeat.
- Remove supernatant and add 5 mL of freshly prepared bleach/NaOH solution (2.5 mL household bleach, 1 mL 10N NaOH, 6.5 mL H2O). Incubate for 5 minutes at RT until the worms break open. Be sure to vortex gently every minute. Monitor the progress of the reaction under the dissecting microscope.
- As soon as all the adults dissolve, add 5 mL of M9 buffer to neutralize the reaction.
- Wash the eggs three times with 10 mL M9 buffer by centrifuging 2 min at 2500 rpm (1100 x g).
- Wash the eggs once with 10 mL S-complete by centrifuging 2 min at 2500 rpm (1100 x g).
- Remove the supernatant, add 10 mL S-complete and transfer the solution to a fresh 50 mL conical tube. Add 30 mL of S-complete to a final volume of 40 mL. Gently shake the tube overnight at room temperature on a nutator or similar device.
Day -2: Tuesday (week 2), 12:00 noon: Seed the animals into plates
- Under the dissecting scope, check whether the worms hatched during the night. Determine the concentration of worms in the S-complete solution by counting the number of worms in 10 μL drops using a dissecting scope. Count at least 10 drops for each sample.
- Re-suspend the worms at a concentration of 80-100 worms/ mL in S-complete. Add Carbenicillin (stock 100 mg/ mL) to a final concentration of 50 μg/ mL and Amphotericin B (stock 250 μg/ mL) to a final concentration of 0.1 μg/ mL. Shake on a nutator. If preparing larger quantities of worms, use a 600 mL nunclon flask with filter cap.
- At 2:30 p.m. add the OP50 prepared in Part 1 to a final concentration of 6 mg/ mL (= 1.2 x109 bacteria/ mL). Return the OP50 to 4°C.
- Transfer 120 μL of the worm/OP50 solution into each well of a 96 well plate. Use 96 well plates with a transparent bottom. Make sure to keep the worms in suspension while pipetting.
- Seal the plate using a tape sealer to avoid contamination and evaporation. Shake the plate on a microtiter plate shaker for 2 min and incubate for 2 days at 20°C until the animals reach the L4 stage.
Day 0: Thursday (week 2) before noon: Sterilize animals by adding Fluorodeoxyuridine (FUDR)
- To sterilize the animals add 30 μL of a 0.6 mM FUDR stock solution to each well. This step brings the final volume in each well to 150 μL and reduces the final concentration of OP50 from 6mg/ mL to 5 mg/ mL (1×109 bacteria/ mL). Seal the plate using tape sealers and shake it for 2-3 min on a microtiter plate shaker. If the OP50 was added at 2:30 on day -2, it is important that the FUDR is added before noon. Return plates to the 20°C incubator
Day 1: Friday (week 2): Add drugs to culture
- By 9:00 a.m. most of the animals should be gravid and contain several eggs each. Add the drugs whose effect on lifespan is to be tested at the desired concentration. If dissolving the drugs in DMSO the final concentrations of DMSO should not exceed 0.6%, as DMSO concentrations higher than 0.6% shorten C.elegans lifespan. After addition of the drug, seal the plates with tape sealer and shake it for 2-3 min on a microtiter plate shaker. Return plates to the 20°C incubator
- Adding the drugs can occasionally kill a few animals per plate, especially if using a solvent other than water. Use an inverted microscope to check for dead animals. Generally, there should be less than 10 dead animals per 96 well plate. Return plates to the 20°C incubator.
Day 4: Monday (week 3): Change sealers
- To allow fresh oxygen to enter the culture remove the sealer, wait 1minute and reseal the plate. Shake the plate for 2-3 minutes on a microtiter plate shaker. Repeat once every week.
Day 5: Tuesday (week 3): Add new OP50 to prevent starvation
- Add 5 μL of the 100 mg/ mL OP50 solution that was prepared in part 1 to each of the 96 wells to prevent starvation.
3. Scoring of Lifespan.
This section describes how survival of the synchronized worm population of Part 2 is monitored until the animals died. To observe the animals in the 96 well plates, use an inverted microscope with a 2x or 2.5x objective. Record survival data three times a week; Monday, Wednesday and Friday. Use movement to determine whether the animals are alive or dead. Strong light, especially blue light, induces the animals to move. Do not remove dead animals from the assay. Occasionally, animals that did not move and were determined dead in the preceding count might move later on.
- On day 0, at the beginning of the experiment count the total number of worms in each well. Censor wells that contain more than 18 animals from the analysis as animals in these wells will not have enough OP50 and will show effects of dietary restriction.
- In order to increase the chance that the animals move shake the 96 well plate on a microtiter plate shaker for 2 min. before counting.
- In each counting session, record date and number of animals which move as live animals. Movement in liquid is much easier than on solid media and can be induced by strong lights. The use of a higher magnification may help to spot very subtle movements like those of the tip of the pharynx. Such subtle movements are often the only movement observed in very old animals.
- Return plates to the 20°C incubator
- Repeat step 2-4 every two to three days until all the animals are dead.
4. Representative Results.
This section shows an example of how to keep records of the lifespan data generated by this assay and some representative results.
Figure 1A shows an example of how to record lifespan data during this assay. An excel sheet is used to keep track of the survival the populations in each well. For each well it’s coordinate in the plate, strain, drug, concentration of the drug and the total number of animals alive on day 0 (X0) is recorded at the beginning of the experiment. Record date, as well as the number of living and dead animals three times a week in order to follow the survival of the various populations in each well. To graph the results, calculate the fraction of animals alive for each day and plot it as a function of time in days. P-values should be calculated using statistical packages like STATA or similar software.
The medium lifespan of C.elegans is temperature dependent. Figure 1B shows that temperature dependent changes in lifespan are accurately reproduced by the microtiter based lifespan assay22. Similarly, the microtiter plate assay reproduces changes in lifespan from mutants reported to have lifespans that differ from wild type N2 animals23-24 25(Fig 1C).
The assay also can be used to make more quantitative statements. In Fig1D mean lifespan is plotted as a function of Mirtazepine concentration7. Each data point represents the mean lifespan of 7-12 population, each living in a different well. Even though the number of animals per well is relatively low (5-15 animals) the well-to-well variation is relatively small as can be seen from the error bars.
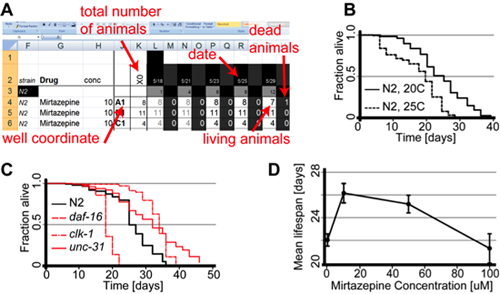
Figure 1. The microtiter plate based lifespan assay accurately reproduces changes in lifespan reported in the literature.
(A) Sample data collected in an excel spreadsheet. During each counting session date, coordinate of the well and the number of live and dead animal was recorded. At the beginning of the experiment, the total number of animals in each well was determined. (B) Survival curve of wild type (N2) animals grown at 20°C and 25°C. (C) Survival curves of strains carrying mutations that affect lifespan. All four strains were assayed in parallel at 20°C. (D) Dose response curve of Mirtazepine treated N2 animals. Mean lifespan is plotted as a function of Mirtazepine concentration. Error bars indicate the S.E.M of 8 wells per condition.
5. Materials.
M9 buffer, 1000 mL
- 6 g Na2HPO4
- 3 g KH2PO4
- 5 g NaCl
- 0.25 g MgSO4∙7H2O
- Add deionized water to 1000 mL
- Autoclave
Potassiumphosphate buffer pH 6.0, 1000 mL
- 136 g KH2PO4
- Add deionized water to 900 mL
- Adjust pH to 6.0 with 5M KOH
- Add deionized water to 1000 mL
- Autoclave
Trace metal solution
- 1.86 g Na2EDTA
- 0.69 g FeSO4 ∙7H2O
- 0.20 g MnCl2 ∙ 4H2O
- 0.29 g ZnSO4 ∙7H2O
- 0.016 g CuSO4
- 1000 mL deionized water
- Autoclave and store in the dark.
S-basal medium, 1000 mL
- 5.9 g NaCl
- 50 mL of 1M potassium phosphate, pH 6.0
- 1000 mL deionized water
- Autoclave
- Let the solution cool and then add 1 mL of 5mg/ mL cholesterol (dissolved in Et-OH).
Potassium citrate 1M, 1000 mL
- 268.8 g tripotassium citrate
- 26.3 citric acid monohydrate
- Add 900 mL deionized water
- Adjust pH to 6.0 with 5M KOH
- Add deionized water to 1000 mL
- Autoclave
S-complete medium, 1000 mL
- 977 mL S-basal
- 10 mL 1M potassium citrate pH 6 (sterile)
- 10 mL Trace metals solution (sterile)
- 3 ml 1M CaCl2 (sterile)
- 3 mL 1M MgSO4 (sterile)
NGM agar
- 3.0g NaCl
- 2.5g Pepton (from casein, pancreatic digest)
- 17g Agar
- Add deionized water to 975 mL and a stirring bar
- Autoclave
- After autoclaving cool down to 55°C and add the following components:
- 0.5 mL of 1M CaCl2 (sterile)
- 1 mL of 5mg/ mL Cholesterol in ethanol
- 1 mL of 1M MgSO4 (sterile)
- 25 mL Potassiumphosphate buffer, pH 6.0 (sterile)
TB, 1000 mL
- 12g Bacto Tryptone
- 24 g Yeast Extract
- 4 mL Glycerol
- Add 900 mL deionized water
- Autoclave
- After autoclaving cool down to 55°C and add the following component:
- add 100 mL of 0.17M KH2PO4/0.72M K2HPO4
0.6 mM Fluorodeoxyuridine (FUDR, sigma cat# F0503), 1000 mL
- 100 mg FUDR
- Dissolve in 670 mL sterile S-complete, make 10 mL or 45 mL aliquots.
- Store at -20 °C.
100 mg/ mL Carbenicillin, 10 mL
- 1 g Carbenicillin
- 10 mL sterile deionized water
- Sterile filtrate and aliquot
- Store at -20 °C.
- Use at a final concentration of 50 μg/ mL
250ug/ mL Amphotericin B, 4 mL
- 1mg Amphotericin B
- 4 mL Et-OH
- Store at -20 °C
- Use at a final concentration of 0.1 μg / mL
Discussion
The protocol presented allows the measurement of C.elegans lifespan in 96 well microtiter plates. As shown in the representative results section it reliably replicates previous findings and provides quantitative information. Using this assay we have successfully screened over 89,000 molecules for their effect on C.elegans lifespan.
For the purpose of drug-screening, measuring lifespan in a 96 well microtiter plate format has several advantages over the classical solid media assay. It reduces the labor required for media preparation, the amount of incubation space, and the amount of drug required. The 96-well format and the microscopy setup allow automation of the entire assay for high throughput screenings.
During the development of the assay multiple values for each variable and combinations thereof were tested for their effects on C.elegans lifespan. These tests included different OP50 concentration ranging from 3 to 10 mg/ mL (3, 4, 6, 8, 10 mg/ mL), different number of worms per well ranging from 7 to 45 (7, 10, 15, 22, 45 worms/well), different culture volumes ranging from 40 to 150 μL per well (40, 60, 80, 100, 120, 150 μL), and changes in buffer composition. If fed with a Carbenicillin-resistant OP50, neither Carbenicillin nor Amphotericin B in the concentrations indicated were found to affect C.elegans lifespan. Continuous gentle shaking of the plates, as is often recommended in C.elegans liquid culture, was found dispensable for the small volumes used in this assay. Gentle shaking however is required if microtiter plates with larger wells are to be used. The values indicated in this protocol have been carefully selected based on statistical comparison of the different conditions tested.
The most surprising feature of this assay is probably the fact that C.elegans can be kept in 96 well plates that are sealed with tape. Side-by-side comparisons did not reveal any difference in lifespan of animals cultured in unsealed plates, in plates sealed with a plastic sealer, or in plates sealed with sealers that allow air exchange. In all three conditions, the animals developed very homogenously from L1 to gravid adults within 65 hours and showed very comparable lifespans. Animals grown in parallel on NGM developed slightly faster, but this difference was independent of the absence or presence of a sealer.
The presented protocol is based on live bacteria, but can be adapted for dead bacteria. However, in a liquid assay a single surviving bacteria can quickly multiply and re-populate the culture. In our hands, the only reliable way to kill bacteria to the extent that they can be used for liquid culture is by prolonged treatment of the bacteria with gamma irradiation.
One important point to consider in the planning of a lifespan experiment is the power of detection. Dependent on the size of the effect, the effect of the drug of interest must be tested in multiple wells. In a typical assay 4 drug-treated and 4 control wells, corresponding roughly to 40-50 animals each, should suffice to detect a 30% increase in lifespan in more than 95% of the experiments. Increases of 14 % are only detected in 60% of the cases and therefore require more replicate wells.
The wealth of lifespan data generated by this assay may be used to develop experiment or strain specific parametrical Gompertz models1. These Gompertz models are useful to determine the power of detection and to estimate the number of false positives and negatives for large-scale screens. We verified the predictions of these models in blind experiments and used them to estimate the numbers of false negatives in large screens (unpublished results).
In summary, we anticipate that the presented assay will be of great use to identify small molecules that extend lifespan of C.elegans and to study the underlying mechanisms.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This Protocol was originally developed at Fred Hutchinson Cancer Research Center in Seattle by Xiaolan Ye and Michael Petrascheck in the Laboratory of Linda Buck. The detailed version above has been prepared to make the entire procedure available to the wider community. We thank Carol Taylor, Sarah LeBoeuf and Andy Tomacelli for critically reading the protocol. This is manuscript #2866 from The Scripps Research Institute. The Petrascheck Lab is funded by the Novartis ADI program.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Amphotericin B | Calbiochem | cat# 171375 | Also called Fungizone | |
| FUDR | Sigma-aldrich | cat# F0503 | FUDR is inactivated by heat, thaw in cold water | |
| Mianserin | Sigma-aldrich | M2525-250MG | Use as positive control at 50μM final concentration | |
| Cyproheptadine | Sigma-aldrich | cat#C6022-MG | Alternative positive control at 10 μM final concentration | |
| Sealing Tape | Nunc | cat# 236370 | Polyester, non-sterile | |
| 96 well plate | Falcon | cat# 351172 | Non-treated, transparent, sterile individual packaged, polystyrene | |
| Nunclon flask | Nunc | cat# 178883 | used for large volumes |
Riferimenti
- Johnson, T. E. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 249, 908-912 (1990).
- Kenyon, C. J. The genetics of ageing. Nature. 464, 504-512 (2010).
- Finch, C. E., Ruvkun, G. The genetics of aging. Annu Rev Genomics Hum Genet. 2, 435-462 (2001).
- Wilson, M. A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 5, 59-68 (2006).
- Evason, K., Huang, C., Yamben, I., Covey, D. F., Kornfeld, K. Anticonvulsant medications extend worm life-span. Science. 307, 258-262 (2005).
- McColl, G. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J. Biol. Chem. 283, 350-357 (2008).
- Petrascheck, M., Ye, X., Buck, L. B. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 450, 553-556 (2007).
- Srivastava, D. Reserpine can confer stress tolerance and lifespan extension in the nematode C. elegans. Biogerontology. 9, 309-316 (2008).
- Wood, J. G. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 430, 686-689 (2004).
- Evason, K., Collins, J. J., Huang, C., Hughes, S., Kornfeld, K. Valproic acid extends Caenorhabditis elegans lifespan. Aging Cell. 7, 305-317 (2008).
- Onken, B., Driscoll, M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 5, e8758-e8758 (2010).
- Wu, Z. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell Mol Biol (Noisy-le-grand). 48, 725-731 (2002).
- Kang, H. L., Benzer, S., Min, K. T. Life extension in Drosophila by feeding a drug. Proc. Natl. Acad. Sci. U. S. A. 99, 838-843 (2002).
- Avanesian, A., Khodayari, B., Felgner, J. S., Jafari, M. Lamotrigine extends lifespan but compromises health span in Drosophila melanogaster. Biogerontology. 11, 45-52 (2010).
- Melov, S. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 289, 1567-1569 (2000).
- Benedetti, M. G. Compounds that confer thermal stress resistance and extended lifespan. Exp. Gerontol. 43, 882-891 (2008).
- Braungart, E., Gerlach, M., Riederer, P., Baumeister, R., Hoener, M. C. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener Dis. 1, 175-183 (2004).
- Kwok, T. C. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 441, 91-95 (2006).
- Moy, T. I. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem Biol. 4, 527-533 (2009).
- Kaletta, T., Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 5, 387-398 (2006).
- Chung, C. T., Niemela, S. L., Miller, R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. U. S. A. 86, 2172-2175 (1989).
- Klass, M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 6, 413-429 (1977).
- Ailion, M., Inoue, T., Weaver, C. I., Holdcraft, R. W., Thomas, J. H. Neurosecretory control of aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 96, 7394-7397 (1999).
- Kenyon, C., Chang, J., Gensch, E., Rudner, A., Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature. 366, 461-464 (1993).
- Lakowski, B., Hekimi, S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science. 272, 1010-1013 (1996).

