Morris Water Maze Test for Learning and Memory Deficits in Alzheimer's Disease Model Mice
Summary
The Morris Water Maze is a behavioral task to test hippocampal-dependent learning and memory. It has been widely used in the study of neurobiology, neuropharmacology and neurocognitive disorders in rodent models.
Abstract
The Morris Water Maze (MWM) was first established by neuroscientist Richard G. Morris in 1981 in order to test hippocampal-dependent learning, including acquisition of spatial memoryand long-term spatial memory 1. The MWM is a relatively simple procedure typically consisting of six day trials, the main advantage being the differentiation between the spatial (hidden-platform) and non-spatial (visible platform) conditions 2-4. In addition, the MWM testing environment reduces odor trail interference 5. This has led the task to be used extensively in the study of the neurobiology and neuropharmacology of spatial learning and memory. The MWM plays an important role in the validation of rodent models for neurocognitive disorders such as Alzheimer’s Disease 6, 7. In this protocol we discussed the typical procedure of MWM for testing learning and memory and data analysis commonly used in Alzheimer’s disease transgenic model mice.
Protocol
1. Preparation
- Equipment preparation
- Obtain a circular pool with a diameter of 150cm and a depth of 50cm (Fig. 1). If using black mice, a white pool should be used; if using white mice, a black pool should be used.
- Arrange the room such that the animal being tested cannot see the experimenter during testing. This can be accomplished with drapes or room dividers.
- Place high contrast spatial cues about the room, and/or on the interior of the pool at a location which would be above the water surface.
- Place a 10cm diameter platform in the pool – white for a white pool, clear plexiglass for a black pool. Fill the pool with water until the platform is 1cm above the water surface. Let the water equilibrate to room temperature (22 °C). Depending on the water temperature this may take one to three days, or alternatively hot water can be added to speed up the equilibration.
- Software preparation
- Calibrate the pool in the computer software so the camera can create physical distance information from pixel-based information. Divide the pool into 4 quadrants. Specify the platform zone as a variable zone which can change with each trial. Create 5 platform subzones – one in each quadrant, and one in the center of the pool. Save the calibration and use it for the remaining test days. (See example Fig. 2).
- Set the maximum trial time as 60 sec. If the mouse finds the platform before this time, program the software to stop the trial when the platform is found.
- Specify the program to begin tracking automatically, when the experimenter exits the testing area. Utilize any “reflection minimization” options your software package provides.
- Track path length, escape latency, and time spent in each quadrant.
2. Day 1: Visible Platform
- Computer Program
- Load the pool calibration into the tracking software.
- Create 5 trials, with an inter-trial interval appropriate for your experiment. Program the platform location and starting direction to differ with each trial. See Table 1 for an example protocol.
- Testing procedure
- Transfer the mice from their housing facility to the behavior room. Keep the mice in an area where they cannot see the pool or spatial cues. Let them adjust to the new environment for at least 30 minutes before testing.
- Place a flag on the platform to increase its visibility.
- To begin testing, lift mouse from the home cage by the base of the tail. Support the mouse as you bring it to the testing area. Lifting the mouse by the base of the tail, gently place the mouse into the water, facing the edge of the pool. Quickly leave the testing area.
- If the mouse finds the platform before the 60 sec cut-off, allow the mouse to stay on the platform for 5 seconds then return it to its home cage. If the mouse does not find the platform, place the mouse on the platform and allow it to stay there for 20 sec before returning it to its home cage.
- Repeat for all mice in the trail. Begin each subsequent trial with a different platform location and starting direction, as you have programmed into your software.
- When testing is complete, return the mice to their housing facility. Mice are dried off and normothermia is assured prior to returning to animal facility.
- In preparation for the following day, remove the flag from the platform and add additional water to the pool to submerge the platform to 1cm below the surface.
3. Days 2-5: Hidden Platform
- Computer Program
- Load the pool calibration into the tracking software.
- Create 5 trials, with an inter-trial interval appropriate for your experiment. Program the platform location to remain in the same position throughout all trials and days, but have the starting direction differ with each trial, each day.
- Testing procedure
- For black mice, add non-toxic, white, powdered tempera paint to the pool and mix thoroughly. Use enough paint such that the submerged platform is not visible from the surface of the water. For white mice, a black pool with clear water and a clear plexiglass platform should be used.
- Follow steps 2.2.3 to 2.2.6.
4. Day 6: Probe Trial
- Computer Program
- Load the pool calibration into the tracking software.
- Create 1 trial with no platform zone, and one starting direction. The starting direction farthest from the platform quadrant used on days 2-5 is preferred. Set the trail length to 60 sec.
- Testing procedure
- Remove the platform from the pool.
- Follow steps 2.2.3 to 2.2.6.
5. Data analysis
- For each day and each mouse, average the 5 trials to give a single path length and escape latency for each test subject. Calculate the combined error appropriately. For day 6, simply collect the path length, escape latency, and time spent in the platform quadrant for each mouse.
- If any differences exist between groups on Day 1, it is likely a problem with vision rather than learning and memory. Only proceed with analysis if no differences are seen on day 1.
- Compare the learning curves for Days 2-5 using statistics appropriate for your data set. A steeper curve represents faster task acquisition; a shallower curve represents a deficit in task acquisition. The data from day 2 to day 5 are analyzed using ANOWA.
- For day 6, compare the percent of time spent in the previously learned platform quadrant, using statistics appropriate for your data set. A higher percentage of time spent in the platform quadrant is interpreted as a higher level of memory retention.
6. Representative Results
We have used the Morris Water Maze test to examine hypoxia’s effect on AD pathogenesis (7) and valproic acid (VPA)’s pharmaceutical potential for AD treatment (6) in transgenic AD model mice. Figure 3 is the representative result we reported in our study on VPA’s effect on memory deficits in the APP23 AD mouse model (6). On day 1 (visible platform trials), there is no difference between the VPA treated and control groups in latency (Fig. 3A) and path length (Fig. 3A) indicating that both of the groups have similar motor and visual capabilities. From this we assume the mice are able to see the flagged-platform and the cues in surrounding environment, and can swim acceptably. For days 2-5 (Day 1 to 4 of hidden platform trials) the example shows a difference in the escape latency (Fig. 3C) and path length (Fig 3D) between the groups, suggesting that VPA treated mice performed significantly better than controls over time. The probe trail results on the last day (Day 6) show that the number of times the mice traveled into the third quadrant, where the hidden platform was previously placed, was significantly greater with VPA treatment compared to control (Fig. 3E). These data indicate that VPA treatment significantly improves the memory deficits seen in APP23 mice.

Figure 1. Equipment setup for the Morris Water Maze visible platform test day. The pool is shielded from the experimenter using room dividers. Spatial cues are located on the walls, and maybe placed on the interior of the pool, above the water surface, if desired. The pool is filled with clear water, with the platform located 1cm above the surface. A flag has been placed on the platform to enhance visibility.
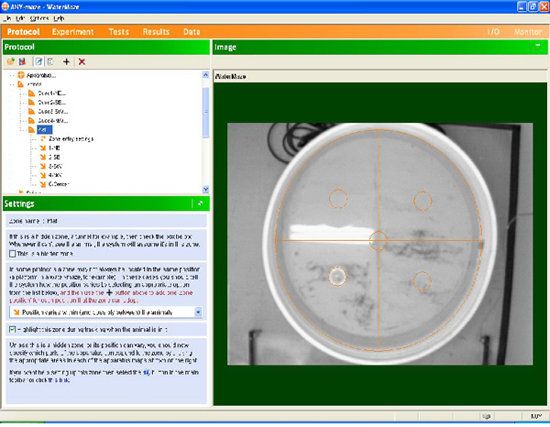
Figure 2. Screen capture from the Any-Maze™ Video Tracking System demonstrating pool calibration. The pool is viewed from above by a black and white analog tracking camera with a RTV24 Digitizer. Several zones are defined within the software and the total pool is divided into 4 quadrants. A fifth, platform zone is entered which can vary across trials, with five possible locations: NW, NE, SW, SE, or Center. A calibration line (ticked line across center) is added to allow the software to convert pixel distances into physical distances.
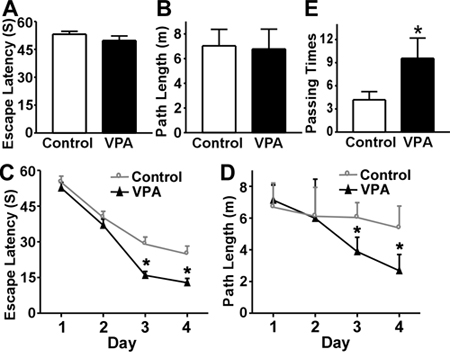
Figure 3. Representative results for the Morris Water Maze. The 7-month APP23 transgenic mice carrying human Swedish mutant APP gene were tested after one month of daily VPA (n=30 mice) or vehicle solution (n=30 mice) injections. (A) During the first day of visible platform tests, the VPA treated and control APP23 mice exhibited a similar latency to escape onto the visible platform. P>0.05 by student’s t-test. (B) The VPA-treated and control APP23 mice had similar swimming distances before escaping onto the visible platform in the visible platform test. P>0.05 by student’s t-test. (C) In hidden platform tests, VPA treated APP23 mice showed a shorter latency to escape onto the hidden platform on the 3rd and 4th day, P<0.001 by ANOVA. (D) The VPA-treated APP23 mice had a shorter swimming length before escaping onto the hidden platform on the 3rd and 4th day, P< 0.01 by ANOVA. (E) In the probe trial on the 6th day, the VPA-treated APP23 mice traveled into the third quadrant, where the hidden platform was previously placed, significantly more times than controls. * P<0.005 by student’s t-test. (Adapted and reprinted from The Journal of Experimental Medicine 205, 2781-2789, 2008, Rockefeller University Press, Originally published in J. Exp. Med. doi:10.1084/jem.20081588.) (6).
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | ||
| Platform location | Starting Direction | Platform Location: SW Starting Location as follows: | No platform. | ||||
| Trial 1 | SW | S | W | N | N | E | N |
| Trial 2 | NW | N | S | W | E | S | |
| Trial 3 | NE | S | N | E | W | W | |
| Trial 4 | Center | E | E | W | S | E | |
| Trial 5 | SE | W | S | S | N | N | |
Table 1. Sample water maze protocol*
* Note how both the platform position and starting direction change on day 1, whereas on days 2-5 the platform position remains constant while the starting direction changes. On day 6, there is no platform and a single trial. The starting direction for day 6 is farthest from the previous platform location (SW) so that the mice must travel some distance before entering the previously learned platform quadrant.
Discussion
Age, sex, species, and strain differences influence MWM performance (8). Studies indicate that aged mice have poor performance in the MWM, while male rodents perform better than females; additionally, floating is more pronounced in mice than rats (9, 10). Therefore, these elements should be equated across all tests. Evidence also suggests that stressed animals perform more poorly in the MWM (11), thus environmental factors which may cause stress, such as temperature, light, and noise, should be monitored and kept constant over the task.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR), the Townsend Family, and Jack Brown and Family Alzheimer’s Research Foundation (to W. S.). W. S. is the holder of the Canada Research Chair in Alzheimer’s Disease. P.L. was supported by a NSERC Alexander Graham Bell Canada Graduate Scholarship Doctoral Research Award and a Michael Smith Foundation for Health Research Senior Graduate Studentship.
Materials
| Name of the reagent | Company | Comments (optional) |
|---|---|---|
| AnyMaze Video Tracking System | Stoelting Company | |
| Tempera Paint | Reeves & Poole Groups | White, powdered |
Riferimenti
- Morris, R. G. M. Spatial localization does not require the presence of local cues. Learning and Motivation. 12, 239-260 (1981).
- O’Keefe, J. A review of the hippocampal place cells. Prog Neurobiol. 13, 419-439 (1979).
- Scoville, W. B., Milner, B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 20, 11-21 (1957).
- Eichenbaum, H., Stewart, C., Morris, R. G. Hippocampal representation in place learning. J Neurosci. 10, 3531-3542 (1990).
- Block, F. Global ischemia and behavioural deficits. Progress in Neurobiology. 58, 279-295 (1999).
- Qing, H., He, G., Ly, P. T., Fox, C. J., Staufenbiel, M., Cai, F., Zhang, Z., Wei, S., Sun, X., Chen, C. H. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med. 205, 2781-2789 (2008).
- Sun, X., He, G., Qing, H., Zhou, W., Dobie, F., Cai, F., Staufenbiel, M., Huang, L. E., Song, W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. , 18727-18732 (2006).
- D’Hooge, R., De Deyn, P. P. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 36, 60-90 (2001).
- Brandeis, R., Brandys, Y., Yehuda, S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 48, 29-69 (1989).
- Lipp, H. P., Wolfer, D. P. Genetically modified mice and cognition. Curr Opin Neurobiol. 8, 272-280 (1998).
- Sandi, C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plast. 6, 41-52 (1998).

