Derivation of Glial Restricted Precursors from E13 mice
Summary
This protocol outlines the derivation of Glial Restricted Precursors from fetal spinal cords and maintained in vitro either for transplantation or for the study of oligodendrocytic lineage.
Abstract
This is a protocol for derivation of glial restricted precursor (GRP) cells from the spinal cord of E13 mouse fetuses. These cells are early precursors within the oligodendrocytic cell lineage. Recently, these cells have been studied as potential source for restorative therapies in white matter diseases. Periventricular leukomalacia (PVL) is the leading cause of non-genetic white matter disease in childhood and affects up to 50% of extremely premature infants. The data suggest a heightened susceptibility of the developing brain to hypoxia-ischemia, oxidative stress and excitotoxicity that selectively targets nascent white matter. Glial restricted precursors (GRP), oligodendrocyte progenitor cells (OPC) and immature oligodendrocytes (preOL) seem to be key players in the development of PVL and are the subject of continuing studies. Furthermore, previous studies have identified a subset of CNS tissue that has increased susceptibility to glutamate excitotoxicity as well as a developmental pattern to this susceptibility. Our laboratory is currently investigating the role of oligodendrocyte progenitors in PVL and use cells at the GRP stage of development. We utilize these derived GRP cells in several experimental paradigms to test their response to select stresses consistent with PVL. GRP cells can be manipulated in vitro into OPCs and preOL for transplantation experiments with mouse PVL models and in vitro models of PVL-like insults including hypoxia-ischemia. By using cultured cells and in vitro studies there would be reduced variability between experiments which facilitates interpretation of the data. Cultured cells also allows for enrichment of the GRP population while minimizing the impact of contaminating cells of non-GRP phenotype.
Protocol
Introduction
In this protocol we show how to extract, select and plate glial restricted precursor (GRP) cells from the spinal cord of E13 mouse fetuses. These cells are early precursors within the oligodendrocytic and astrocytic cell lineages and are defined by their expression of A2B5. In the GRP medium supplemented with FGF-2, the A2B5+ GRPs will begin expressing the early oligodendrocytic lineage markers PDGFαR and NG21,2. These oligodendrocyte lineage cells pass through a series of distinct phenotypic stages, each of them characterized by morphological changes, as well as expression of markers to specific developmental stages.
These precursor cells are currently being studied as a potential source for cell-based therapeutic approaches in disorders of the central nervous system white matter, including multiple sclerosis, leukodystrophies and periventricular leukomalacia (PVL)3,4,5,6. Studies using human brain derived oligodendrocyte lineage precursors have reported successful remyelination in brains of the shiverer mouse dysmyelination model as well as in demyelinated spinal cord rodent models6,7,8,9. These glial precursor cells have also been used in studies of other white matter disease models such as spinal cord injury and amyotrophic lateral sclerosis10,11,12,13.
Our laboratory has developed an early postnatal hypoxia-ischemia mouse model with which we are evaluating the efficacy of these spinal cord derived GRP cells as a restorative approach in PVL, the leading cause of cerebral palsy14,15,16. There is also a rodent brain derived OPC model that has been widely used for studying white matter injury since the GRP cells have a tendency to differentiate into the astrocytic pathway17. The OPC phenotype has already entered the oligodendrocyte lineage pathway, a phenomenon that seems irreversible. However, we are interested in assessing the response of a wider spectrum of oligodendrocyte progenitors including GRPs and possibly even the NEPs derived at E10.5. For this reason we have adopted the spinal cord derived GRP model for our work.
In addition to the use of GRPs for cell replacement, the derivation of these cells from wild type and transgenic rodent models of white matter diseases allows further study into glial differentiation under normal and disease conditions in an in vitro setting. Previous publications show evidence of selective vulnerability of oligodendrocytic lineage precursor cells to various external stressors like maturation dependent glutamate excitotoxicity, oxidative stress, and select factors implicated in neuroinflammation18,19. Our studies have focused on understanding the cellular and molecular mechanisms behind the development of these white matter injuries, evaluating the susceptibility of cells in the oligodendrocyte lineage spectrum to insult as well as analyzing putative therapeutic approaches.
Materials
All procedures involving animals conform to PHS policy and the JHU IACUC. All procedures should be performed in a laminar flow hood in order to maintain aseptic conditions. Commonly dissections are performed in a horizontal flow hood and in vitro work in a vertical flow hood. All media used cold during dissections. The mice used in our study are a wild type CD-1 strain and a transgenic GFP in a C57/BL6 background. One CD-1 mouse dam typically yields 10+ fetuses which are enough to seed 1-2 T25 flasks. Typically, two dams are sacrificed at a time and fetal spinal cords pooled and plated into 1 T25 flask per dam. Once cells have been derived they can be expanded and characterized in vitro for subsequent studies.
1. Preparation of Media and Flasks
- GRP stock medium is used as the Dissection medium: DMEM / F12 1:1 (Invitrogen) + B27 (50x; Invitrogen) N2 (100x; Invitrogen) Supplements and Bovine Serum Albumin (BSA; 0.5% w/v).
- Dissociation medium: same as dissection medium.
- Culture medium : GRP stock medium + FGF-2 (10-20 ng/ml; Invitrogen) and heparin (1 μg/ml; Sigma).
- PLL/laminin coated 75 cm2 or 25 cm2 flasks (T75 or T25).
- Tissue culture flasks (75 cm2, Falcon) were coated with 15-20μg/ml Poly-L-lysine (PLL; Sigma) in distilled H2O, incubated at 37 °C for 1 hr then aspirated. Flasks are washed 1X with PBS then coated with 15-20 μg/ml Laminin (Sigma) in PBS at 37 °C for 1 hr, then aspirated and fresh PBS or media added until cells are ready to be plated. Following coating, flasks should never be allowed to dry but can be kept in PBS or media at 4 °C for brief periods before use.
2. Instruments and Other Material
- Petri dishes: 4 per dam: 3 Petri dishes for dissection (75 mm), a 20 mm dish for collecting the harvested spinal cords.
- Large scissors (43 mm Operating scissors, Roboz) and tissue Forceps for opening the dam s abdomen.
- Small scissor for dissecting the uterus and removing the fetuses (15 mm Micro Dissecting Scissors, Roboz).
- Micro Dissecting spring scissors (6 mm) to dissect the spinal cord of the fetuses.
- Micro Dissecting angled Forceps for anchoring the dam and fetal body during surgery and later removing the spinal cord once exposed.
- 40 or 70 micrometer cell strainer for removing cell debris before plating.
- 0.05% Trypsin prewarmed to 37 °C.
- 10 mg/ml DNAse 1 (Sigma), prepared as a 100x stock (1 g/ml).
- Bio-hazard bag for animal corpses.
- 15 and 50 ml centrifuge tubes for processing extracted spinal cords.
3. Determining Timed Pregnancy
Pregnant mouse dams are either ordered from commercial sources specifying delivery on embryonic day E12 or E13 of fetal development or pregnancy determined in-house. Briefly, two females are placed together with one male in the late afternoon and left overnight together. The next day the females are observed for the presence of the vaginally located mucus plug. The mucus plug is only an indication that mating occurred so animals are subsequently weighed daily to monitor rate of weight increase. The day the mucus plug is observed is defined as embryonic day one (E1).
4. Tissue Derivation
- Work under a horizontal flow hood to maintain aseptic conditions.
- Spray down the hood and working area with 70% ethanol.
- Autoclave instruments or spray liberally with 70% ethanol.
- Prepare instruments, media, and microscope for use.
- Pour 20 ml of cold dissection medium into sterile Petri dish.
- Anesthetize animal with chloral hydrate (500 mg/kg) administered intraperitoneally (IP) followed by cervical dislocation or decapitation.
- Spray the abdominal area of dam with 70-90% ethanol (disinfection)
- Open abdomen with large scissor and forceps.
- In CD1 and C57/BL6 strains: Usually 8 to 14 fetuses will be inside the uterus. The number of fetuses is strain dependent.
- Extract the mouse uterus including the pups and place in fresh cold dissection medium in a clean Petri dish to wash blood and tissue debris from fetus.
- Extract each fetus from the uterine horn and separate them from the embryonic sac and placenta using the small scissors. Transfer the extracted fetuses into fresh dissection medium in a new Petri dish.
- Dissection medium should be at low temperature to lower metabolic activity and preserve viability of derived cells.
- Work from now on with two forceps and micro dissecting spring-scissors.
- Working under dissection microscope, cut the skin along the spinal cord with the micro surgical scissors and peel skin back to expose the spinal cord.
- Transect the spinal cord with the microsurgical scissors at about C1 and at the beginning of the tail.
- Remove the spinal cord with blunt forceps, ensure all bone and cartilage are removed and transfer to 3rd Petri dish of dissecting medium.
- To prevent overgrowth of meningeal tissue in subsequent cell cultures try to remove as much of the meninges as possible from the spinal cord. Due to the nascent protruding peripheral nerves this is more difficult than removing meningeal tissue from extracted cerebrum.
- Once meninges have been removed, transfer spinal cords to 20 mm Petri dish
- Clean up by thoroughly spraying the area with 70-90% ethanol.
- The following steps for dissociation should be done quickly to maintain a high viability of the derived spinal cord tissue.
5. Culture Establishment
- Trypsin should be pre-warmed for at least 30 min in a 37 °C H2O-bath. An appropriate aliquot should be removed from the stock to reduce the exposure of stock to temperature fluctuations.
- Transfer the harvested spinal cords to a 50 ml centrifuge tube containing 10ml pre-warmed trypsin (0.05%; Quality Biologicals) and 100 μl DNAse (10 mg/ml) and triturate briefly.
- Incubate in 37 °C H2O-bath for 10 minutes.
- Triturate and incubate for another 10 min.
- Add 5 ml GRP medium (defined above) and centrifuge for 5 min at 1000 RPM.
- Aspirate supernatant and resuspend pellet with 10 ml GRP medium and add DNAse-1 (10 mg/ ml; Sigma).
- Incubate in 37 °C H2O-bath for 10 minutes.
- Centrifuge 5 min at 1000 RPM and aspirate supernatant.
- Resuspend pellet with fresh GRP medium then filter debris with a 40 – 70 μm cell strainer.
- Plate on PLL/laminin coated 25 cm2 flasks (T25) and incubate at 37 °C, 95% humidity and 5% CO2.
- 100% media change on following day.
- At this point, GRPs have attached and started extending processes.
6. Immunopanning the GRP Population
- Plate spinal cord tissue in PLL/laminin coated flasks until flask is 85-90% confluent or about a week.
- Harvest cells by scraping flask with a rubber policeman or with 0.05% trypsin, triturate thoroughly but gently, centrifuge at 1000 RPM for 5 min and thoroughly resuspend pellet with 3 ml GRP medium.
- Transfer 1 ml each to 3 T25 flasks or 3 ml to 1 T75 flask add appropriate volumes of medium to completely cover cells and allow flasks to reach 80-90% confluence.
- Change media every other day, sooner if medium depletes quickly.
- Coat Bacteriological Petri dishes (75 mm) overnight at 4 °C with an anti-mouse IgM antibody (Southern Biotech), 10 μg/ml in PBS.
- Aspirate the excess buffer and wash the Petri dishes 3x with PBS.
- Add A2B5 antibody (Millipore) at a concentration of 5 μg/ml diluted in PBS and incubate for 1 hr at room temperature (RT).
- Wash plates again 3x with PBS then add 8 ml of GRP medium to prevent drying of plate.
- Transfer 5 ml of GRP medium from Petri dishes and harvest GRP cells by scraping flasks with a rubber policeman to detach all cells
- Triturate cell suspension briefly to break up clumps and transfer 5ml cell suspension to the A2B5 coated bacteriological Petri dishes.
- Incubate 1 hr at room temperature without shaking.
- After 1 hr aspirate media and wash plates 8x with PBS.
- Gently scrape cells with a rubber policeman in 2 ml GRP media then transfer to PLL/ laminin coated plates or flasks with appropriate volume of GRP medium.
7. Freezing and Storage of Derived Cells
- Cells are harvested from an 85-90% confluent T25 or T75 flask with 2 ml 0.05% trypsin.
- Trypsin is diluted and inactivated with 8ml GRP stock medium and centrifuged at 1000 RPM for 5 min.
- Pellets from T25 flasks are resuspended in 1 ml GRP freezing medium, (GRP stock medium supplemented with 10% (v/v) DMSO and optionally, 20% FBS). The larger pellets from T75 flasks are diluted two-fold.
- Cell suspensions of 1.5 — 3 x 1010,11,12,13 cells are transferred to cryogenic vials (Nalgene) and stored overnight in a cryogenic container (Nalgene) containing isopropanol at -80 °C to slowly freeze overnight.
- Following overnight freezing cells are transferred to freezer boxes and stored 6 months to 1 yr at -80 °C or longer term in N(l). Thawed cells are typically 60-80% viable depending largely on the quality of the PLL/laminin coating.
8. Representative Results
CO2 is not used for anesthetizing the animal because of the possible downstream effect on the viability of the fetuses and ultimately the derived cells. Furthermore, it will be very difficult to totally remove the meninges from the spinal cord during the derivation procedure but the combination of GRP medium and immunopanning will eliminate these fast growing cells. Similarly, the entire spinal cord is harvested despite a greater ventral concentration of the GRP population due to its originating in the ventral spinal cord and migrating dorsally20. However the GRP medium selects for the GRP phenotype and that population is maximized by A2B5 immunopanning. After plating spinal cord tissues, the in vitro cultures are incubated for two passages or about a week. After 2 days in culture, cells of different morphologies can be observed in the tissue culture flasks indicating the heterogeneity of the population but the GRP medium selects for the GRP phenotype. These cells are a combination of A2B5+ GRP, E-NCAM+ neuronal restricted precursors (NRP) and nestin+, A2B5- neuroepithelial cells and possibly fibronectin+ meningeal cells. In addition, more mature phenotypes may be present expressing markers including doublecortin and Tuj1 for neuronal lineage, GFAP for astrocytes and PDGFaR and GalC for oligo lineage In order to maximize a highly GRP enriched population from the starting heterogeneous pool (Figure 1A), cells can be double-immunopanned to select and eliminate the E-NCAM+ cells followed by selection for the A2B5+ GRP population once cell density has increased to about 85-90% confluency in T75 flasks. These GRP cells maintain their ability to become astrocytes despite being in a medium that selects for the oligodendrocyte phenotype so subsequent A2B5 immunopanning may be necessary to maintain a highly enriched GRP population. Immunopanning generally yields up to 95% A2B5+ cells but for even greater yield A2B5 conjugated magnetic beads (Miltenyi Biotec Inc.) can be used to provide a yield of up to 97%. Vigilance in the maintenance GRP cultures such as regular media changes will minimize the proliferation of non-GRP cell types. Even in the absence of BMP-4 astrocytes may still be found and depleted media in particular seems to induce differentiation to an astrocytic phenotype. Although the B27 supplement contains tri-iodothyronine hormone (T3), there has been no observed tendency to differentiate into oligodendrocytes in the GRP population, only when higher levels of T3 are added to the medium does this phenomenon occur. However, a T3 free B27 supplement can be substituted if there is a concern for contamination by mature oligodendrocytes (Figure 1B). These GRP cells can be readily identified by their morphology of small soma with 2 or 3 short processes but to screen for other cell types that may be present, immunocytochemistry can be performed for the previously named common developmental and cell type specific markers. There will commonly be a small persistent population of A2B5- cells that are neuroepithelial (NEP) and capable of becoming either GRP or NRP. Fortunately, the longer cells are maintained in the GRP medium the more likely that medium will select for the GRP phenotype.
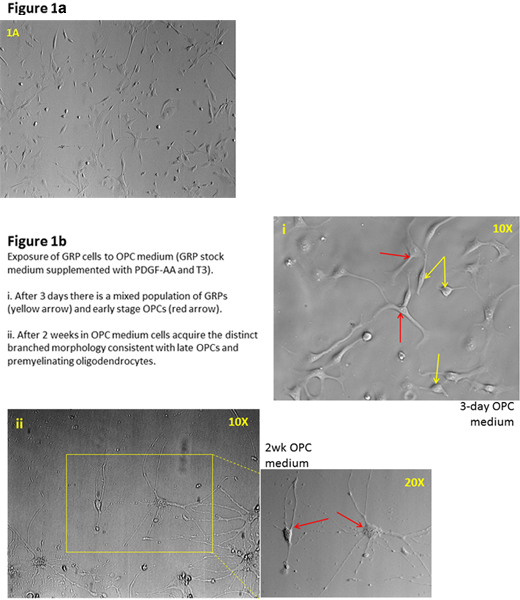
Figure 1. a) Plated spinal cord cells of heterogeneous morphology are observed after 2 days in culture. b) Immunopanning maximizes a highly GRP-enriched population from the starting heterogeneous pool.
Discussion
Disorders of central nervous system white matter include a large number of etiologies, including genetic, inflammatory, ischemic and toxic causes19,21,22,23,24. While multiple sclerosis and vasculopathies are the leading cause of white matter disease in adults, periventricular leukomalacia associated with prematurity is the most common cause of white matter injury in the childhood population. In order to further delineate disease mechanisms further study of cells ofoligodendrocytic lineage, which are greatly affected by the perinatal insult, is required. GRPs were isolated from the rodent embryonic spinal cord and shown to be tripotential in nature, giving rise to oligodendrocytes and two distinct astrocyte populations, but do not differentiate into neurons2,25,26. Likewise,oligodendrocyte precursor cells (OPC) derived from rat optic nerve and late embryonic or early postnatal rat brain have been demonstrated to mature into functioning oligodendrocytes in the appropriate media conditions27,28.Additionally, fetal or adult derived human OPC have been further manipulated into more mature oligodendrocyte phenotypes29,30.
GRP cells have been isolated from both the spinal cord of E13.5 rats and E12-13.5 in mice. In the rat differences have been reported between dorsal- and ventral-derived GRP cells in their response to conditions that promote generation of oligodendrocytes, or astrocytes with ventral-derived GRPs exhibiting a greater propensity to differentiate into the more mature phenotypes25,26,28. This is may be due to the ventral to dorsal migration of the nascent GRP cells, which could be less receptive to the differentiation cues. Immunopanning allows for the subpopulation that expresses A2B5 to be isolated and collected for maintenance in culture.
Our mouse GRP cultures are derived from total spinal cord tissue that yields a heterogeneous, pooled population of cells from which GRP cells are selected by chemical (medium) and antibody based techniques. While our studies have utilized the spinal cord derived mouse GRP cells, it has more recently been reported the derivation of brain derived mouse oligodendrocyte progenitor cells that are also capable of developing into mature oligodendrocytes31. Here the derived cells are initially cultured as oligospheres that using the appropriate culture medium are manipulated into an enriched PDGFαR+, OPC population31. Our studies have focused on the effect of our model of hypoxic-ischemic insult on cells in the oligodendrocyte lineage spectrum from A2B5+, PDGFαR- GRP precursors to the myelin basic protein expressing (MBP+) mature oligodendrocytes. Indeed our in vitro characterization has shown these immature GRP cells mature into MBP expressing cells either in monocultures or in co-cultures with cortical neurons. These cells can be manipulated in culture into specific differentiation pathways for studying the mechanisms involved in perinatal white matter injury. There are several mouse models of white matter diseases involving cells of oligodendrocyte lineage and the availability of this population allows for thorough investigation into the mechanisms that cause injury as well as to explore the usefulness of possible cell therapy options.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We wish to thank Dr. Devin Gary for his insight and feedback on the use of GRP cells.
Materials
| Name of the reagent | Company | Catalogue number |
|---|---|---|
| DMEM/ F12 1:1 | Invitrogen | 11320033 |
| B27 | Invitrogen | 17504044 |
| N2 | Invitrogen | 17502048 |
| rH-FGF-basic | Invitrogen | PHG0026 |
| Trypsin (0.05%) EDTA | Quality Biological, Inc. | 118-087-721 |
| POLY-L-LYSINE HBr | Sigma | P1274-25MG |
| Laminin | Sigma | L2020-1MG |
| Dnase 1 | Sigma | DN25 |
Riferimenti
- Kalyani, A., Hobson, K., Rao, M. S. Neuroepithelial stem cells from the embryonic spinal cord: isolation, characterization, and clonal analysis. Developmental biology. 186, 202-202 (1997).
- Rao, M. S., Mayer-Proschel, M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Developmental biology. 188, 48-48 (1997).
- Goldman, S. A., Lang, J., Roy, N. Progenitor cell-based myelination as a model for cell-based therapy of the central nervous system. Ernst Schering Research Foundation workshop. (60), 195-195 (2006).
- Goldman, S. A., Schanz, S., Windrem, M. S. Stem cell-based strategies for treating pediatric disorders of myelin. Human molecular genetics. 17, 76-76 (2008).
- Goldman, S. A., Windrem, M. S. Cell replacement therapy in neurological disease. Philosophical transactions of the Royal Society of London. 361, 1463-1463 (2006).
- Walczak, P., All, A. H., Rumpal, N. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 59, 499-499 (2011).
- Liu, S., Qu, Y., Stewart, T. J. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proceedings of the National Academy of Sciences of the United States of America. 97, 6126-6126 (2000).
- Windrem, M. S., Schanz, S. J., Guo, M. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell stem cell. 2, 553-553 (2008).
- Groves, A. K., Barnett, S. C., Franklin, R. J. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 362, 453-453 (1993).
- Back, S. A., Han, B. H., Luo, N. L. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 22, 455-45 (2002).
- Johnston, M. V., Trescher, W. H., Ishida, A. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatric research. 49, 735-735 (2001).
- Lepore, A. C., Han, S. S., Tyler-Polsz, C. J. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron glia biology. 1, 113-113 (2004).
- Rothstein, J. D., Dykes-Hoberg, M., Pardo, C. A. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 16, 675-675 (1996).
- Johnston, M. V., Ferriero, D. M., Vannucci, S. J. Models of cerebral palsy: which ones are best. Journal of child neurology. 20, 984-98 (2005).
- Comi, A. M., Johnston, M. V., Wilson, M. A. Strain variability, injury distribution, and seizure onset in a mouse model of stroke in the immature brain. Developmental neuroscience. 27, 127-127 (2005).
- Comi, A. M., Weisz, C. J., Highet, B. H. A new model of stroke and ischemic seizures in the immature mouse. Pediatric neurology. 31, 254-254 (2004).
- Dietrich, J., Noble, M., Mayer-Proschel, M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 40, (2002).
- Alberdi, E., Sanchez-Gomez, M. V., Marino, A. Ca(2+) influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes. Neurobiology of disease. 9, 234-234 (2002).
- Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet neurology. 8, 110-110 (2009).
- Warf, B. C., Fok-Seang, J., Miller, R. H. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J. Neurosci. 11, 2477-2477 (1991).
- Deng, W., Poretz, R. D. Oligodendroglia in developmental neurotoxicity. Neurotoxicology. 24, 161-161 (2003).
- Deng, W., Rosenberg, P. A., Volpe, J. J. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proceedings of the National Academy of Sciences of the United States of America. 100, 6801-6801 (2003).
- Karadottir, R., Cavelier, P., Bergersen, L. H. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 438, 1162-1162 (2005).
- Pleasure, D., Soulika, A., Singh, S. K. Inflammation in white matter: clinical and pathophysiological aspects. Mental retardation and developmental disabilities research reviews. 12, 141-141 (2006).
- Gregori, N., Proschel, C., Noble, M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J. Neurosci. 22, 248-248 (2002).
- Rao, M. S., Noble, M., Mayer-Proschel, M. A tripotential glial precursor cell is present in the developing spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 95, 3996-3996 (1998).
- Raff, M. C., Miller, R. H., Noble, M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 303, 390-390 (1983).
- Strathmann, F. G., Wang, X., Mayer-Proschel, M. Identification of two novel glial-restricted cell populations in the embryonic telencephalon arising from unique origins. BMC developmental biology. 7, 33-33 (2007).
- Nunes, M. C., Roy, N. S., Keyoung, H. M. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature. 9, 439-439 (2003).
- Roy, N. S., Wang, S., Harrison-Restelli, C. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J. Neurosci. 19, 9986-9986 (1999).
- Chen, Y., Balasubramaniyan, V., Peng, J. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nature protocols. 2, 1044-1044 (2007).

