Preparation of Acute Subventricular Zone Slices for Calcium Imaging
Summary
A method to load subventricular zone (SVZ) cells with calcium indicator dyes for recording calcium activity is described. The postnatal SVZ contains tightly packed cells including neural progenitor cells and neuroblasts. Rather than using bath loading we injected the dye by pressure inside the tissue allowing better dye diffusion.
Abstract
The subventricular zone (SVZ) is one of the two neurogenic zones in the postnatal brain. The SVZ contains densely packed cells, including neural progenitor cells with astrocytic features (called SVZ astrocytes), neuroblasts, and intermediate progenitor cells. Neuroblasts born in the SVZ tangentially migrate a great distance to the olfactory bulb, where they differentiate into interneurons. Intercellular signaling through adhesion molecules and diffusible signals play important roles in controlling neurogenesis. Many of these signals trigger intercellular calcium activity that transmits information inside and between cells. Calcium activity is thus reflective of the activity of extracellular signals and is an optimal way to understand functional intercellular signaling among SVZ cells.
Calcium activity has been studied in many other regions and cell types, including mature astrocytes and neurons. However, the traditional method to load cells with calcium indicator dye (i.e. bath loading) was not efficient at loading all SVZ cell types. Indeed, the cellular density in the SVZ precludes dye diffusion inside the tissue. In addition, preparing sagittal slices will better preserve the three-dimensional arrangement of SVZ cells, particularly the stream of neuroblast migration on the rostral-caudal axis.
Here, we describe methods to prepare sagittal sections containing the SVZ, the loading of SVZ cells with calcium indicator dye, and the acquisition of calcium activity with time-lapse movies. We used Fluo-4 AM dye for loading SVZ astrocytes using pressure application inside the tissue. Calcium activity was recorded using a scanning confocal microscope allowing a precise resolution for distinguishing individual cells. Our approach is applicable to other neurogenic zones including the adult hippocampal subgranular zone and embryonic neurogenic zones. In addition, other types of dyes can be applied using the described method.
Protocol
1. Preparation of Solutions, Dissection, and Vibratome
- Solutions must be prepared at the correct osmolarity and pH (Table 1). Compared to artificial cerebrospinal fluid (ACSF), dissection solution is prepared with lower concentrations of sodium and calcium, and higher concentrations of magnesium. This is to minimize excitotoxicity effects during slicing.
- Both dissection and ACSF solutions must be saturated with 95% O2/5% CO2 by bubbling for at least 10 min to reach the desired pH of 7.3.
- Prepare an ice bath in a tray. Place a dissection plate in the ice bath and fill with dissection solution. Bubble the dissection plate.
- Prepare the vibratome by creating an ice bath. Place the sectioning dish in the ice bath, fill the dish with dissection solution, and bubble.
- Fill the slice holding chamber with ACSF and bubble to ensure that solution is adequately aerated regardless of where slices are placed.
2. Removal of Brain Tissue
Acute brain slices tend to be healthier with younger mice. The postnatal SVZ’s cellular architecture in rodents is mature around postnatal day (P)201. Therefore, we try to limit our experiments to mouse ages of P20-P30 but we have performed successful experiments in mice as old as 3 months. Throughout handling of the tissue, one must be mindful to minimize mechanical movements and pressure to the brain, maintain cooled conditions, and expose the SVZ to solution as quickly as possible following sacrifice.
- After injection of pentobarbital, the mouse is rapidly decapitated. The fur is removed and incisions are made through the base of the skull. The head is then placed in a tray filled with dissection solution.
- While stabilizing the head with forceps, continuous cuts are made around the skull and sometimes up the midline with microscissors. Be careful to minimize contact with the tissue. Since the olfactory bulb is the final destination of newborn neurons from the SVZ, one must be mindful of preserving that region, especially during bone removal around this area. Microscissor cuts made around the skull should be on the dorsal side, to allow for easy removal of the skull.
- Removal of the skull overlying the brain is now separated from the bones underneath the brain. If cuts in the previous step are made correctly, this overlying bone can be removed easily with tweezers or forceps.
- While still holding the head with forceps, one can use a surgical blade to cleanly remove several pieces of brain tissue prior to slicing, such as the following. A coronal cut can be made at the level of lambda. Sagittal cuts can be made on both sides of the brain lateral to the SVZ. A conservative estimate would be ~2.5 mm from the midline. If desired, the brain can also be hemisected here. These cuts will both facilitate mounting of the tissue and allow the SVZ to be reached more quickly when brain slicing. It is important to remember to exert the minimal amount of mechanical forces on the brain (e.g. not pushing or pulling).
3. Acute Brain Slice Preparation
- To prepare for subsequent steps, ensure that the super glue used to mount the tissue is free of clogs and ready to apply to the vibratome plate. It is important to go as fast as possible without damaging the brain.
- The brain tissue can then be scooped from underneath, which separates the tissue from underlying bone while simultaneously separating nerves.
- The tissue is mounted and glued on a plate and placed on the vibratome. Due to the rostral-caudal anatomy of the SVZ, we have focused on making sagittal slices as it preserves the migratory pathway of neuroblasts, the glial sheath, and the vasculature that are largely aligned in this direction. One can either mount the tissue at the midline or on the flat surface created by making sagittal cuts lateral to the SVZ. An optional step is to place a 3% agarose block behind the tissue to serve as an abutment to the blade as the slices come off. We preferentially used cyanoacrylate-based super glue.
- Rinse the blade with ethanol to remove oils and rinse with distilled water. Place the blade on the blade holder. Mount the blade holder to the vibratome.
- Adjust vibratome settings to cut tissue at a thickness of 250-350 μm per slice. It is important to cut using high frequency vibrations and low speed.
- Section progressively through the tissue. The appearance of the olfactory bulb is a good indicator that one is near the SVZ in sagittal slices as the most lateral slices will contain neither region. Other landmarks to look for include the ventricle and the thickening of the corpus callosum, beneath which the SVZ is located. The SVZ will appear first in more lateral sagittal slices. The RMS will show in medial sagittal slices.
- Remove slices containing the SVZ with a plastic Pasteur pipette with the tip cut off. Transferring to the slice holding chamber. We often obtain more slices than we can use in a work day. However, slices will vary on the amount of cells containing the SVZ. It is sometimes necessary to look through several slices to find one desired for imaging.
4. Preparation of Microscope and Dyes
The slices require a one hour incubation time in ACSF to recover from the dissection and slicing. Several steps can be performed during this slice recovery period.
- Preparation of pipettes for calcium dye and/or drug application
- Glass pipettes need to have a tip of 2-3 μm in diameter and a tip length (~2 mm) and angle (~16° for the pipette holder) that prevents contact with the perfusion chamber and allows room for placement of the objective.
- One can typically prepare 6-8 pressure pipettes for each day of experiments.
- Calcium dye preparation
- When preparing the working solution (4-5 μM by bath, 250 μM by pressure application), it is best to expose the minimum amount of DMSO to cells. This can be achieved by making highly concentrated stocks. For example, for Fluo-4, one tube containing 50 μg is used per day. One can make a 10 mM stock of this aliquot by adding 4.6 μl of Pluronic F-127 20% solution in DMSO.
- Preparing microscope setup (Figure 2).
- Use of a peristaltic pump for solution flow has helped minimize image drift. Run the peristaltic pump prior to allow ACSF to run through the perfusion lines and ensure that it is bubble-free.
- Set the tube inlet on the perfusion chamber and ensure there are no leaks.
- Position the vacuum tip to ensure that the solution is being aspirated from the perfusion chamber. Ensure that it is at an adequate level so that the objective is immersed at the proper working distance, but not so high such that solution may spill over the chamber. A low level of solution will also minimize flow distortions. A constant aspiration can also be verified by listening for steadiness.
5. Dye Loading
This method has been described in detail in another manuscript2. Please see figures therein.
- Bath loading dye
- Make a 1-2 ml working solution of the dye. For Fluo-4 AM, a concentration of 4-5 μM is adequate for labeling SVZ neuroblasts.
- Slices are first transferred to a 35 mm culture dish with a mesh bottom that is already filled with ACSF. Replace the solution by using a 3 ml plastic transfer pipette to gently remove the ACSF, while simultaneously adding the calcium dye working solution.
- Incubate at 37 °C for 30-60 min in a 5% CO2 gas-controlled environment.
- Place the slice back into the ACSF-filled holding chamber to wash away dye that has not entered cells. Alternatively, one can place it directly on the microscope perfusion chamber that is running ACSF. This wash period is 30-45 min.
- Transfer the slice to the perfusion chamber.
- Pressure application of dye
- Transfer the slice from the holding chamber to the perfusion chamber on the microscope setup.
- Fill a glass pipette with a working solution that is 250 μM and place on the micromanipulator.
- Lower the pipette to the region of interest. The pipette can be placed such that the tip is either several micrometers above the slice surface or buried several micrometers below the slice surface, but away from the recorded region. The angle of the pipette did not affect the loading efficiency. When imaging below the slice surface, we find that placing the pipette tip below the imaging plane will label our desired imaging area better.
- When pressure applying the dye, set the Picospritzer so that it is <3 psi. We have not estimated the volume of dye diffusion, because this is subject to variability of the pipette tip diameter, pressure applied, and density of tissue where the dye is injected. We apply simply until we achieve our desired loading as assessed by eye. Minimize damage to the slice during application, especially when the tip is placed beneath the slice surface. Apply for 1-2 min. Sometimes repeated applications are necessary depending on labeling as assessed by eye. For this concentration and application duration, we find that surface application of dye preferentially labels neuroblasts while below the surface application preferentially labels astrocytes. However, >3 min application at 500 μM will also label neuroblasts regardless of whether the tip is above or below the slice surface (JC Platel, unpublished observation). Allow for wash and slice recovery for 30-45 min.
6. Confocal Imaging of Calcium Activity
- Find the region of interest first with a lower-power objective such as 4x or 10x. Place in the center of the field before switching to a higher power objective. At this point, one can assess general slice health by noting the appearance of SVZ cells under differential interference contrast (DIC). Healthy cells appear round and plump. In addition, healthy cells will display faint Fluo-4 loading as supposed to no loading at all or bright fluorescence (a dying cell).
- Lower the water-immersion objective onto the region of interest, which is now dye-loaded. Either a 40x or 60x objective has been adequate for our needs, although 40x provides a wider field of view and allows for greater pipette access. When imaging below the surface, we often go at least 15 μm below the slice surface where the 3-dimentional architecture and cell health are better preserved.
- Verify that perfusion and vacuum are working adequately.
- Setup the microscope so that time-lapse resolution and spatial resolution are set to desired rates. For confocal microscopy, these factors must be balanced as an increase in temporal resolution usually results in a loss of spatial resolution due to the scanning nature of the system. For calcium activity, we have imaged a frame approximately every second with a 512×512 pixel resolution. If multiple channels acquired separately are necessary, this will also affect acquisition rates. Set duration of movie (2-10 min).
- Initiate run. If performing pharmacological studies, wash on antagonists or non-desensitizing agonists for at least 5-10 min, and then make another movie of 5-10 min. Agonists that may desensitize receptors can be acutely applied, such as by using a pressure pipette controlled by a micromanipulator.
7. Calcium Analysis
Analysis follows standard procedures that we described in pervious publications2-4. F0 (i.e. baseline) and F are the mean fluorescence intensities measured throughout all of the regions of interest (ROIs) and in each ROI, respectively. A change in fluorescence was considered to be a Ca2+ increase if it was >15% F/F0 increase. Intracellular Ca2+ changes were calculated using Calsignal5.
8. Representative Results
We have been able to obtain selective loading of SVZ cells depending on the dye loading protocols described above and elsewhere2-4,6. Bath loading or pressure application on the slice surface of Fluo-4 AM (4-5 or 250 μM, respectively) labels mostly neuroblasts. While pressure application can load neuroblasts more quickly than bath loading in a single slice, bath loading allows the simultaneous loading of multiple slices. We confirm the identity of labeled cells as neuroblasts by (1) whether they display migratory behavior4,7, (2) their morphology, (3) negative staining of sulforhodamine 101, an astrocyte-specific dye3 or (4) positive staining with DCX-DsRed expression (JC Platel, unpublished observation). Neuroblasts migrate quickly (average of 60 μm/hr) at physiological temperature4,7-9, but their movement can be readily observed even at room temperature. The movement of neuroblasts does not pose an issue with regards to placing regions-of-interest (ROI) to track calcium activity since our movies are typically short in duration. However, during long waiting periods, such as during drug solution exchanges, investigators must be careful to match ROIs in control and treatment periods. It is not uncommon for neuroblasts to migrate into or out of the imaging field or focal plane.
Pressure application of Fluo-4 AM (250 μM) deep into the slice and for limited duration (<2 min) preferentially labels SVZ astrocytes2. Astrocyte labeling has been verified by positive labeling with sulforhodamine 101 and their morphology, especially by the presence of projections and endfeet on blood vessels which we have recently described3. Using these methods, we have observed spontaneous activity in both the SVZ neuroblast and astrocyte population (Figure 1). The activity in astrocytes often takes the form of waves that engage blood vessel3. Furthermore, using calcium imaging as an assay, the application of pharmacological agonists has revealed or confirmed expression of GABAA receptors on neuroblasts and astrocytes6, AMPA and NMDA receptors on neuroblasts4.
Figures
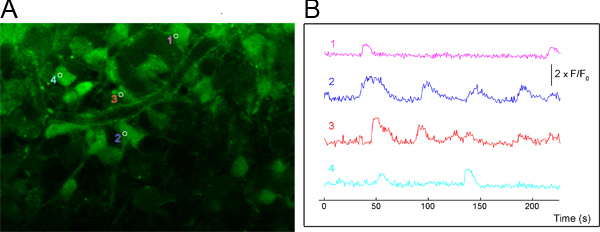
Figure 1. Propagating calcium activity in astrocytes. (A) Representative averaged image from a time-lapse recording of calcium activity. Astrocytes in the SVZ were loaded with pressure application of Fluo-4 AM deep into the slice. Movies were acquired at 0.75 s time-steps. Regions of interest (ROI) are placed over cells exhibiting activity. (B) Traces from ROIs placed over cells from the movie depicted in (A). Traces were filtered with a moving average and normalized. The vertical scale represents 2 x ΔF/F0 where F is the signal intensity and F0 is the average baseline signal and ΔF= F-F0.
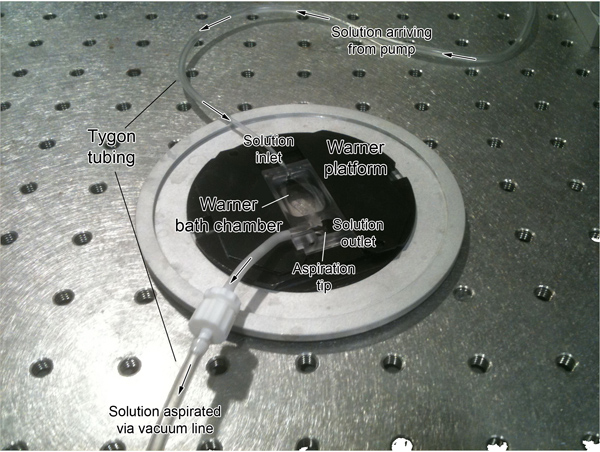
Figure 2. Picture of the perfusion system. One end of a tube is submerged in 95% O2/5% CO2 gas-perfused solution. The solution is then perfused through the tube and to the bath chamber by a peristaltic pump (not shown). The other end is inserted into the solution inlet of the bath chamber mounted on a platform. The aspiration tip is then connected to the solution outlet of the chamber and set at a level to determine solution height. From the outlet, the aspiration tip is connected to the vacuum line, which aspirates the solution from the chamber into a waste container. The perfusion system is shown off the microscope stage for visual clarity.
Discussion
Calcium imaging of SVZ cells has been used to study patterns in spontaneous activity in neuroblasts10, receptor channel expression in both neuroblasts and astrocytes4,6,8 and astrocytic calcium waves3. Since cells in the SVZ are either immature or have glial properties, they do not fire action potentials11,12, meaning that millisecond changes in voltage potential indicative of activity in mature networks is not applicable in this region. Therefore, capturing the slower calcium events (on the order of seconds) is not only biologically meaningful, but perhaps the most relevant form of activity in these cells.
Investigators must be mindful of many steps in this procedure, especially with regards to slice health. Improper making of solutions, poor water quality (used to make solutions), slow and imprecise dissections, and the use of dirty razor blades to cut tissue could all have detrimental effects on activity.
With regards to the use of calcium indicators, while we only discussed the use of Fluo-4AM, we have tried other commercial dyes, including Oregon Green BAPTA-AM, which has a higher baseline loading level than Fluo-4. We chose to focus on Fluo-4 because of its larger dynamic range compared to other dyes, but other investigators may find different dyes advantageous for their purposes. Every dye may have different affinity for certain cell types. Concentration and method of loading may need to be adjusted for each dye. We preferentially used pressure loading to label SVZ astrocytes and healthier, deeper cells. A financial factor to consider is that using pressure application is more costly than bath application, although the dye solution in the pressure pipette can be frozen and reused the next day. Alternatively, genetically-encoded calcium indicators (GECIs), such as GCaMP3, have been increasingly popular for studying other systems and brain regions13,14. These GECIs have the advantage of being driven under cell-specific promoters, but their kinetics and dynamic range are generally not better than the organic dyes15. Their use in the postnatal SVZ also requires viral labeling or electroporation of the construct, the latter limiting study of neuroblasts to the neonatal period due to loss of plasmid during cell division.
Slice drift prevents reliable signal acquisition during the duration of the movie and is one of the most challenging technical hurdles with live-imaging experiments of brain slices. It is affected by several factors including perfusion rate, vacuum placement, objective weight, and, if applicable, temperature gradients. If temperatures greater than 25 °C are necessary for experiments, one should attempt to heat solutions and perfusion chambers at the lowest temperature where they can address their questions since temperatures may lead to significant, undesired focus drift. Objective heaters and heated enclosures could also help minimize this effect, although we have tried the former without much success.
Barring these technical hurdles, researchers have the opportunity to assay a large number of cells in a postnatal developing region. This presents the opportunity to address activity on a population level, which could yield new insights into the processes regulating neurogenesis.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the NIH (DC007681, A.B.), CT Stem Cell grant (A.B.), Pardee foundation (A.B.), Predoctoral Ruth L. Kirschstein National Research Service Awards (NRSA) (S.Z.Y.), and an NSF Graduate Research Fellowship (B.L.). We thank the Bordey lab members for helpful comments on the manuscript. The present material is based upon work partly supported by the State of Connecticut under the Connecticut Stem Cell Research Grants Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Connecticut, the Department of Public Health of the State of Connecticut or CT Innovations, Incorporated.
Materials
| Solute | Company | Catalog Number | Dissection (mM) |
| Sucrose | Sigma | S0389 | Dissection: 147 mM ACSF: 0 mM |
| NaCl | Sigma | S9888 | Dissection: 42 mM ACSF: 126 mM |
| KCl | Sigma | P3911 | Dissection: 2.5 mM ACSF: 2.5 mM |
| MgCl2.6H2O | Sigma | M9272 | Dissection: 4.33 mM ACSF: 1 mM |
| NaH2PO4.H2O | Sigma | S8282 | Dissection: 1.25 mM ACSF: 1.25 mM |
| Glucose | Sigma | G8270 | Dissection: 10 mM ACSF: 10 mM |
| NaHCO3 | Sigma | S6014 | Dissection: 26 mM ACSF: 26 mM |
| CaCl2.2H2O | Sigma | C3306 | Dissection: 1.33 mM ACSF: 2 mM |
Table 1. Chemical list and recipes of dissection solution and ACSF.
| [header] | |||
| Name | Company | Catalogue Number | Comments |
| Vibratome | Leica | VT 1000S | |
| Super Glue | Surehold or 3M | Surehold 3G Super Glue or 3M Vet-Bond | |
| Dissection tools | Roboz or Ted Pella | ||
| Fluo-4 AM calcium-sensitive dye | Invitrogen | F14201 | |
| Oregon Green BAPTA-1 AM calcium-sensitive dye | Invitrogen | O6807 | |
| Pluronic F-127 20% solution in DMSO | Invitrogen | P3000MP | |
| Upright confocal microscope | Olympus | FV300 or FV1000 | |
| Water-immersion objectives | Olympus | LUMPlanFl 40 x W/IR (NA 0.80); LUMPlanFl 60 x W/I (NA 0.90) | |
| Micromanipulators | Sutter | MPC-200/MPC-325/MPC-385 | |
| Pressure controller | Parker Hannifin | Picospritzer | <3 PSI during application |
| Pipette puller | Sutter or Narshige | Sutter P-97 or Narshige PP-830 | |
| Glass pipettes | Sutter | BF150-110-10 | I.D.:1.10, O.D.: 1.50 |
| Peristaltic pump | Harvard Apparatus | Model 720 | flow rate: 1 ml/min |
| Chamber bath | Warner Instruments | RC-26 GLP | Low profile allows for objective clearance |
| Tubing | Tygon | ||
| Temperature Controller | Warner Instruments | TC-324B/344B |
Table 2. Materials/equipment list.
Riferimenti
- Peretto, P., Giachino, C., Aimar, P., Fasolo, A., Bonfanti, L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J. Comp Neurol. 487, 407-427 (2005).
- Lacar, B., Young, S. Z., Platel, J. C., Bordey, A. Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice. Front. Neurosci. 4, 10-3389 (2010).
- Lacar, B., Young, S. Z., Platel, J. C., Bordey, A. Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur. J. Neurosci. , (2011).
- Platel, J. C., Dave, K. A., Gordon, V., Lacar, B., Rubio, M. E., Bordey, A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 65, 859-872 (2010).
- Platel, J. C., Dupuis, A., Boisseau, S., Villaz, M., Albrieux, M., Brocard, J. Synchrony of spontaneous calcium activity in mouse neocortex before synaptogenesis. Eur. J. Neurosci. 25, 920-928 (2007).
- Young, S. Z., Platel, J. C., Nielsen, J. V., Jensen, N. A., Bordey, A. GABAA increases calcium in subventricular zone astrocyte-like cells through L- and T-type voltage-gated calcium channels. Front. Cell. Neurosci. 4, 8 (2010).
- Bolteus, A. J., Bordey, A. GABA Release and Uptake Regulate Neuronal Precursor Migration in the Postnatal Subventricular Zone. J. Neurosci. 24, 7623-7631 (2004).
- Platel, J., Heintz, T., Young, S., Gordon, V., Bordey, A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in a whole mount preparation of the subventricular zone. J. Physiol. (Lond). 586, 3783-3793 (2008).
- Nam, S. C., Kim, Y., Dryanovski, D., Walker, A., Goings, G., Woolfrey, K., Kang, S. S., Chu, C., Chenn, A., Erdelyi, F., Szabo, G., Hockberger, P., Szele, F. G. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. J. Comp. Neurol. 505, 190-208 (2007).
- Lacar, B. L., Platel, J. C., Bordey, A. GABA controls Ca2+ activity-dependent network synchrony in the adult neurogenic forebrain. , (2007).
- Liu, X., Bolteus, A. J., Balkin, D. M., Henschel, O., Bordey, A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 54, 394-410 (2006).
- Wang, D. D., Krueger, D. D., Bordey, A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J. Neurophysiol. 90, 2291-2302 (2003).
- Tian, L., Hires, S. A., Mao, T., Huber, D., Chiappe, M. E., Chalasani, S. H., Petreanu, L., Akerboom, J., McKinney, S. A., Schreiter, E. R., Bargmann, C. I., Jayaraman, V., Svoboda, K., Looger, L. L. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 6, 875-881 (2009).
- Zhao, Y., Araki, S., Wu, J., Teramoto, T., Chang, Y. F. An expanded palette of genetically encoded Ca(2) indicators. Science. 333, 1888-1891 (2011).
- Shigetomi, E., Kracun, S., Sofroniew, M. V., Khakh, B. S. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 13, 759-766 (2010).

