Assessment of Sensorimotor Function in Mouse Models of Parkinson’s Disease
Summary
In Parkinson’s disease and movement disorders in general, sensitive and reliable behavioral assays are essential for testing novel potential therapeutics. Here, we describe a manageable battery of sensorimotor tests for mice that are sensitive to varying degrees of injury to the nigrostriatal system and useful for preclinical studies.
Abstract
Sensitive and reliable behavioral outcome measures are essential to the evaluation of potential therapeutic treatments in preclinical trials for many neurodegenerative diseases. In Parkinson’s disease, sensorimotor tests sensitive to varying degrees of nigrostriatal dysfunction are fundamental for testing the efficacy of potential therapeutics. Reliable and quite elegant sensorimotor measures exist for rats, however many of these tests measure sensorimotor asymmetry within the rat and are not entirely suitable for the newer genetic mouse models of PD. We have put together a battery of sensorimotor tests inspired by the sensitive tests in rats and adapted for mice. The test battery highlighted in this study is chosen for a) its sensitivity in a wide variety of mouse models of PD, b) its ease in implementing into a study, and c) its low expense. These tests have proven useful in characterizing novel genetic mouse models of PD as well as in testing potential disease-modifying therapies.
Introduction
Parkinson’s disease (PD) is a debilitating neurodegenerative disorder primarily characterized by the progressive loss of dopaminergic neurons in the substantia nigra and the development of Lewy body inclusions in central and peripheral systems. Patients suffer from sensorimotor impairments, including bradykinesia, tremor, rigidity, and postural instability that worsen over time. Although rare, familial forms of the disease discovered over the last 15 years have led to the identification of important novel targets for potential disease-modifying therapeutics. Mutations in genes encoding alpha-synuclein, parkin, DJ-1, LRRK2, and ATP13A2 among others mark the development of a new “generation” of animal models of PD, genetic mouse models.
Excellent non-drug-induced behavioral measures exist for the extensively studied unilateral 6-hydroxydopamine (6-OHDA) rat model of PD. These include tests for limb-use asymmetry, movement initiation, somatosensory neglect, reaching abilities, and more recently ultrasonic vocalizations1-6. These tests are sensitive to varying degrees of nigrostriatal dopamine neuron loss and have been used extensively to evaluate the efficacy of various types of potential therapeutics7-11. However, with the genetic mouse models there is not a clear consensus on the best sensorimotor tests to use or how many to use. This is problematic when characterizing a novel genetic mouse model, in preclinical studies, and when trying to make comparisons between models. Over the past decade we have worked to put together a battery of sensorimotor tests for mice similar to what has been used successfully in rats. The tests described in this article have been used to help characterize numerous genetic mouse models of PD and are currently used in preclinical studies testing novel potential therapeutics12.
The most common tests used to assess motor function in mice are activity in the open field and the rotarod test of coordination13. Although both of these tests are automated, relatively easy to use and provide information on sensorimotor function, they often lack the sensitivity needed to detect subtle alterations in the nigrostriatal dopamine system. For example, parkin deficient mice with subtle alterations in dopamine function do not display impairments on the rotarod but do display motor impairments on a challenging beam test14. In addition, mice treated with moderate doses of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) do not show impairments on the rotarod but do have significant alterations in gait and impairments on an inverted grid test15. Therefore, studies that use only the rotarod for phenotypic assessment may miss more subtle impairments. An optimal approach, in our opinion, to behavioral characterization is one that includes a battery of tests that are sensitive to different aspects of sensory and motor function, and subtle changes in basal ganglia function13-15. Here we describe how to measure and analyze sensorimotor function in mice using a challenging beam traversal test, a test of spontaneous activity in the cylinder, and a response to sensory stimuli test.
Protocol
Optimally mice should be tested during their active (dark) cycle. The mice in our laboratory are maintained on a reverse light/dark cycle that enables us to conveniently test the mice during their active period (and ours). Testing is not started until at least one hour into the dark cycle. However, it is not always possible for an investigator to maintain a separate mouse room with a reverse light cycle. In that case, all three tests can be performed during the light cycle but keep in mind that time to traverse on the beam, number of steps and rears in the cylinder, and contact and removal times may be longer when tested during this time.
All three tests described below can be performed by one experimenter, but for those who are not as experienced handling and working with mice or have little to no experience measuring behavior in mice then an additional experimenter may be needed. In this case the second experimenter can assist with the challenging beam test by placing mice on the beam while the other experimenter records the trial or in the adhesive removal test the second experimenter can run the timer while the other places the sticker on the snout and places the mouse in the cage.
1. Challenging Beam Traversal Procedure
- Invert 3 clean mouse cages on a tabletop or in a mouse cage-changing station. Line the cages up evenly so that they can support the length of the beam (1 meter).
- Assemble the four sections of the beam from the widest to narrowest section and place on top of the inverted mouse cages.
- Place the homecage of the mice that you plan to test on its side and at the end of the beam so that the narrowest end of the beam leads right into the homecage.
- Pick up the first mouse by the base of its tail and support its hindlimbs with the palm of your hand. Place the mouse at the wide end of the beam and let go of its tail and remove your hand.
- Let the mouse sniff and move around in order to become better oriented to the apparatus- if the mouse turns around gently redirect it to the desired direction.
- Lift up the homecage (even if it has cagemates in it) keeping it in the same position on its side and bring it close to the test mouse. As the test mouse begins to try and enter the homecage move it back so that the mouse does not enter but makes a step forward. Continue to do this all the way down the beam. At the end allow the mouse to enter the homecage. Do the same procedure for each mouse in the cage. This is the first “assisted” trial.
- The beam should be thoroughly cleaned between cages with a disinfectant provided by the animal care or veterinary staff. Within a cage it is important to clean off any urine or fecal pellets off the beam before the next mouse is run because this will distract the next mouse.
- Once all of the mice in the cage have gone through one assisted trial then pick up the first mouse again and place it at the wide end of the beam. If needed, with your hand gently orient the mouse in the desired direction and lightly touch its backside to encourage it to move along the length of the beam into its homecage. If the mouse stops to sniff or walk off the beam correct the mouse with your hand or pick it up and place it on the same section where it walked off. Have your hand nearby to guide the mouse to the end of the beam. You want to try and minimize the mouse stopping and exploring while on the beam. The more this is done during training the less the mouse tends to do it during testing.
- The trial ends when the mouse places one of its forelimbs into the homecage. The next mouse can then receive its 2nd trial. Mice receive a total of 5 trials on the first training day, alternating between mice so that each mouse has an intertrial interval of ~30 sec or more. Typically by the last few trials mice will traverse the length of the beam, on their own, without need for correcting.
- 24 hr later day 2 of training can begin. On day 2 the mice receive 5 more trials on the same beam set-up as in day 1. The mice should not need any assistance but may need to be corrected or touched on the backside to prevent stopping or exploring.
- 24 hr later the actual test day can begin. For the test day, the beam is set up in a similar manner to the previous 2 training days. However, now a mesh grid that corresponds to each beam width is placed on top of each beam section. A videocamera is used to record all beam traversal trials and can be performed by one or multiple experimenters.
- Label a card with the essential experiment information (date, mouse #, trial #, etc.). Place this card in front of the camera and record for 2-3 sec so that it is clear which mouse is being tested and what trial it is on.
- The mouse is placed on top of the grid surface on the widest section of the beam and recorded as it moves along the beam. The recording should be close enough so that the entire length of the mouse’s body is visible on the camera. If it is too far then slips are difficult to see and if it is too close then limb movements can be missed. The camera should be positioned so that the grid is in the middle of the viewer.
- On the Test day all mice receive 5 trials on the grid-surfaced beam.
2. Analysis of Beam Video
- Videos can be viewed directly from the camera or connected to a television or computer for a larger screen view. Videotapes should be scored by an experimenter blind to genotype and treatment condition within the experiment.
- Errors for each trial are scored as the video is played in slow motion. Slips or errors are counted when the mouse is facing and moving forward. A slip through or outside of the grid is considered an error when the limb slips beyond 0.5 cm below the grid surface (halfway down). Any slips made after a mouse stops or orients its head to the side are not considered errors. If the mouse is not moving forward and a limb or limbs repeatedly slips through the grid it is NOT considered an error. On the narrow section of the beam the base of the hindlimbs can be on top of the grid and the toes hanging off the side, this is not considered an error.
- Steps are also counted in slow motion. The hindlimb facing the camera is used to track the number of steps. Counting begins when the mouse makes its first step forward and ends when the mouse places its forelimb into the homecage.
- Time to traverse is scored using a stopwatch and is done in real time. The timer is started when the mouse begins to move forward and ends when the first forepaw is placed in the homecage.
3. Spontaneous Activity in the Cylinder Procedure
- Invert 3 clean mouse cages on a tabletop or in a mouse cage-changing station. Arrange two cages next to each other ~18 cm apart so that the front of the cages are facing the experimenter. The remaining cage is placed behind the other two cages and will serve to support the mirror.
- Place the piece of glass on top of the 2 cages and position it so the glass is being supported by the outer edge of the first 2 cages.
- Place the cylinder on top of the glass and the mirror at an angle beneath the glass, leaning on the 3rd cage in back. The angle can range anywhere from 30°- 45° but more importantly the view from the mirror has to include the full diameter of the cylinder.
- Set the videocamera up in front of the mirror and adjust both the mirror and videocamera until you can view the entire diameter of the bottom of the cylinder.
- Set the timer for 3 min and label a card with the important experiment details (date, mouse#, etc.). Videorecord the label for 2-3 sec.
- Place the mouse in the cylinder and press record and the timer. Record the mouse for three minutes. It is important that the testing area is quiet as loud noises and conversation can distract the mouse and potentially cause freezing behavior.
- During the 3 min use a hand counter to count the number of rears the mouse makes while in the cylinder. A rear is defined as a vertical movement with both forelimbs off the floor so that the mouse is standing only on its hindlimbs. At the end of 3 min remove the mouse and place it back into its homecage.
- Clean the cylinder and glass with a disinfectant provided by the animal care or veterinary staff. Allow the cleaning solution to dry before placing the next mouse in the cylinder.
4. Analysis of Spontaneous Activity
- Videos can be viewed directly from the camera or connected to a television or computer for a larger screen view. The videotapes should be scored by an experimenter blind to genotype and treatment condition within the experiment.
- Forelimb steps are counted as the video is played back in slow motion. A forelimb step is counted when an animal moves both forelimbs sequentially across the floor of the cylinder in one consecutive movement. A forelimb movement is not counted if the time between the movement of one forelimb and the other forelimb is longer than 5 sec. Hindlimb steps are counted in the same manner as forelimb steps.
- Time spent grooming is measured using a stopwatch and playing back the video in real time. Grooming bouts on the snout, vibrissae, and body are measured.
5. Adhesive Removal
- Place mouse in its homecage in the testing room or in a mouse cage-changing station.
- Remove the feeder bin from the cage and replace the cage lid. Allow the mice/mouse to habituate to the testing room and the cage without the feeder for 1 hr.
- For testing use a clean cage to place cagemates so the test mouse is the only one in the homecage. Remove ~3/4 of the bedding and place it in the clean cage with the cagemates.
- In the homecage scruff the test mouse in order to restrain it and using a pair of small forceps place one adhesive label onto the snout of the mouse. Gently press the label on the snout with the forcep and release the mouse. Place the lid on the cage and start a stopwatch. When the mouse makes an attempt to remove the label with its forepaws record the time. If the mouse makes contact but does not remove the label then keep the timer going until it does remove it and record that time as well (contact and removal time).
- If the mouse does not contact or remove the sticker within 60 sec then the trial is ended and the sticker is removed manually by the experimenter.
- Place the test mouse in the clean cage and remove the next mouse and place it in the homecage and begin testing. All mice receive 3 trials. It is important to alternate between mice rather than doing all three trials in a mouse at one time. Trials where the label falls off or is not secure on the snout are not counted.
Representative Results
The challenging beam, spontaneous activity in the cylinder, and adhesive removal tests are all highly useful assays of sensorimotor function in mice. We have found alterations in sensorimotor function using these tests in multiple genetic and toxin mouse models of Parkinson’s disease including parkin knockout, parkin Q311X, Pitx3-aphakia, alpha-synuclein overexpressing, and unilateral 6-hydroxydopamine mice14, 16-19. Here we show data collected from mice overexpressing human wildtype alpha-synuclein under the Thy1 promoter (Thy1-aSyn) and Pitx3-aphakia mice 18,20. On the challenging beam we reliably detect increased errors per step in Thy1-aSyn compared to wildtype mice (Figure 1). In the same line of mice we also repeatedly detect a robust decrease in hindlimb stepping in the cylinder (Figure 2)12, 16, 21. In the adhesive removal test Pitx3-aphakia mice with a profound decrease in nigrostriatal dopamine neurons show significantly increased time to contact compared to wildtype controls (Figure 3).
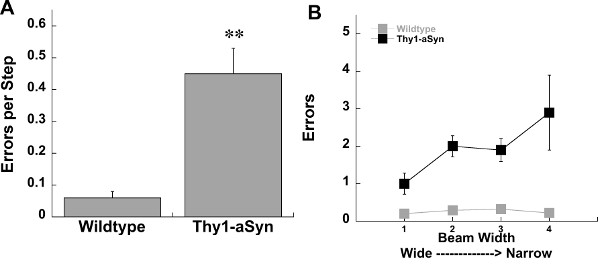
Figure 1. Errors on the challenging in Wildtype (n=13) and Thy1-aSyn (n=4) at 4 months of age. A) Errors per step, mean of five trials. B) Mean errors at each beam width. ** represents p<0.01 respectively compared to Wildtype mice. Student’s t-test.
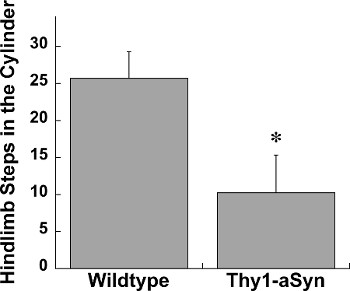
Figure 2. Hindlimb stepping in the cylinder in Wildtype (n=13) and Thy1-aSyn (n=4) mice at 4 months of age. * represents p<0.05 compared to Wildtype. Student’s t-test.
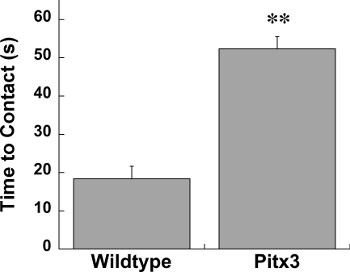
Figure 3. Contact time in Wildtype (n=10) and Pitx3-aphakia (n=12) mice at 4 weeks of age. ** represents p<0.01 compared to Wildtype. Mann-Whitney U.
| Test | Parameters | Analysis Options |
| Challenging Beam | Errors | Beam Widths (Errors only) Mean of 5 Trials Individual Trials |
| Time | ||
| Steps | ||
| Spontaneous Activity | Forelimb Steps | Mean Forelimb/Hindlimb Ratio |
| Hindlimb Steps | ||
| Rears | Mean | |
| Grooming Time | Mean | |
| Adhesive Removal | Contact time | Mean Median Best/Worst Score Removal time Contact time score |
| Removal time |
Table 1. Sensorimotor function Test Battery: Analysis Options.
Discussion
In the present study we show how to perform and analyze three useful tests of sensorimotor function in mice. These include the challenging beam, spontaneous activity in the cylinder, and response to sensory stimuli (adhesive removal). These tests were chosen for the following reasons 1) we and others have found them to be highly sensitive to varying degrees of nigrostriatal dopaminergic dysfunction in genetic mouse models14,16-18, 2) only a short amount of training is required for the beam and handling for the response to sensory stimuli tests and once trained the assays can be performed in one test session, and 3) the price of the equipment needed to perform the tests is quite low compared to purchasing more automated equipment such as the rotarod and open field chambers.
The challenging beam test is not only useful at detecting motor performance and coordination deficits in genetic mouse models of PD but is also useful in uncovering impairments in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated and 6-hydroxydopamine-treated mice and norepinephrine deficient mice19, 23-25. In addition, we find the challenging beam to be less influenced by body weight compared to other tests of motor performance and coordination (rotarod and pole test). This is particularly beneficial when working with aged male mice that can weigh up to 50+ grams. Similar to the beam, alterations in spontaneous activity are reliably observed in parkin knockout, PQ311X, Thy1-aSyn, Pitx3-aphakia, and LRRK2 mice14, 16-18, 26. The adhesive removal test is also sensitive to genetic manipulation including parkin knockout, parkin Q311X, Thy1-aSyn, and DJ-1 knockout mutations in mice14,16, 17, 22. In the unilateral 6-hydroxydopamine-treated mouse the adhesive removal test is performed in a manner similar to how it is typically done with rats1,2. The adhesive label is placed on each forepaw using a forcep and the time to remove the label is recorded. We found that 6-hyrdoxydopamine-treated mice will remove the label from the unaffected limb prior to removing the label on the affected limb 19.
This test battery is easy to implement in aging studies using chronic treatments but it is also easy to use in pharmacological studies. Once animals are trained and ready for testing a test station for each assay can be set up. The animals are then run in the same order on each test. The drug of interest can be administered and once the desired drug peak concentration is reached each mouse can be tested on the beam and then moved to the cylinder for three minutes and then to the adhesive removal test. We found this strategy to work well when testing different dopamine agonists in mice18, 21. Both the beam and adhesive removal tests are amenable to repeated testing, however spontaneous activity in the cylinder is affected by repeated testing resulting in reduced activity over time16. Depending on how robust the deficit is in the mouse you may still be able to detect differences with repeated testing in the cylinder16. In general, habituation and reduced activity is observed as soon as the 2nd exposure to the cylinder however, we find that we can get sufficient activity levels when we run the spontaneous activity test immediately following beam traversal in pharmacological studies18,21. The number of spontaneous activity testing sessions to include in a study will be dependent on mouse strain (some are definitely more active than others) and treatment duration. In our pharmacological studies in mice on a mixed C57BL/6 X DBA background we measured activity between 2-4 times and the testing sessions were separated by one week21.
The beam and cylinder tests do require post-testing analysis of videorecorded behaviors. For this aspect of the analysis it is particularly important to have raters that are blind to the experimental conditions. When we train new raters in the laboratory we have the person score behavior on videotapes previously analyzed by an expert rater from the lab. The criteria are explained and shown to the new rater and then the person begins to score the previously analyzed tapes on their own. The scores are then compared to the expert’ ratings. The person is not allowed to rate new data until they are within 95-98% accuracy of the expert. All data is then randomly spot-checked for accuracy by an expert rater.
The battery of tests described in this study is designed to assess sensorimotor function in mice. However, whenever characterizing a novel mouse model of disease it is always important to do a basic examination of the animal as well. Body weight and temperature should be monitored throughout testing and any abnormal behaviors should be noted, like the removal of vibrissae or clasping of the hindlimbs when picked up by the tail. Basic neurological assessments are described elsewhere in detail27-29.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work is funded by NIH/NINDS NS07722-01 and the Gardner Family Center for Parkinson’s Disease and Movement Disorders.
Materials
| Material Name | Company | Dimensions | Comments |
| Challenging beam apparatus | Starks Plastics | Segment 1= 3.5 cm Segment 2= 2.5 cm Segment 3= 1.5 cm Segment 4= 0.5 cm |
Contact information: 11276 Sebring Dr. Forest Park ,OH 45240 USA Ph +1 (513) 541-4591 Fax +1 (513) 541-6773 |
| Cylinder | Starks Plastics | 15.5 cm diameter 12.7 cm height |
Contact information: 11276 Sebring Dr. Forest Park ,OH 45240 USA Ph +1 (513) 541-4591 Fax +1 (513) 541-6773 |
| Mesh Grid Wiring | Ace Hardware | 1 cm2 | |
| Mouse Cages | Ancare | 19 x 29 x 12.7 cm | |
| Glass | Ace Hardware | 19 cm2 | |
| Mirror | Ace Hardware | 18 x 13 cm | |
| Camcorder | Sony HDR-HC9 | ||
| MiniDV Tapes | Sony DVC premium | 90 min long play | |
| Labels | AveryColor Coding Labels | 6.35 mm diameter (1/4″ Round) |
Riferimenti
- Schallert, T., Upchurch, M., et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacology Biochemistry and Behavior. 16 (3), 455-462 (1982).
- Schallert, T., Upchurch, M., Wilcox, R. E., Vaughn, D. M. Posture-independent sensorimotor analysis of inter-hemispheric receptor asymmetries in neostriatum. Pharmacology Biochemistry and Behavior. 18 (5), 753-759 (1983).
- Schallert, T., Norton, D., Jones, T. A. A clinically relevant unilateral rat model of parkinsonian akinesia. Journal of Neural Transplantation and Plasticity. 3 (4), 332-333 (1992).
- Schallert, T., Tillerson, J. L., Emerich, D. F., Dean, R. L., Sandberg, P. R. Intervention strategies for degeneration of DA neurons in parkinsonism: Optimizing behavioral assessment of outcome. Central Nervous System Diseases. , 131-151 (2000).
- Whishaw, I. Q., O’Connor, W. T., Dunnett, S. B. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 109 (5), 805-843 (1986).
- Johnson, A. M., Doll, E. J., Grant, L. M., Ringel, L., Shier, J. N., Ciucci, M. R. Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J. Vis. Exp. (54), e2835 (2011).
- Connor, B., Kozlowski, D. A., Schallert, T., Tillerson, J. L., Davidson, B. L., Bohn, M. C. Differential effects of glial cell line-derived neurotrophic factor (GDNF) in the striatum and substantia nigra of the aged Parkinsonian rat. Gene Therapy. 6 (12), 1936-1951 (1999).
- Kozlowski, D. A., Connor, B., Tillerson, J. L., Schallert, T., Bohn, M. C. Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Experimental Neurology. 166 (1), 1-15 (2000).
- Yang, M., Stull, N. D., Berk, M. A., Snyder, E. Y., Iacovitti, L. Neural stem cells spontaneously express dopaminergic traits after transplantation into the intact or 6-hydroxydopamine-lesioned rat. Experimental Neurology. 177 (1), 50-60 (2002).
- Luo, J., Kaplitt, M. G., et al. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science. 298 (5592), 425-429 (2002).
- Yasuhara, T., Matsukawa, N., et al. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. Journal of Neuroscience. 26 (48), 12497-12511 (2006).
- Fleming, S. M., Mulligan, C. K., et al. A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions. Journal of Molecular and Cellular Neuroscience. 46 (3), 597-606 (2011).
- Sedelis, M., Schwarting, R. K. W., Huston, J. P. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behavioural Brain Research. 125 (1-2), 109-125 (2001).
- Goldberg, M. S., Fleming, S. M., et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. Journal of Biological Chemistry. 278 (44), 43628-43635 (1074).
- Tillerson, J. L., Caudle, W. M., Reveron, M. E., Miller, G. W. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Experimental Neurology. 178 (1), 80-90 (2002).
- Fleming, S. M., Salcedo, J., et al. Early and progressive sensorimotor abnormalities in mice overexpressing wild-type human alpha-synuclein. Journal of Neuroscience. 24 (42), 9434-9440 (2004).
- Lu, X. H., Fleming, S. M., et al. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. Journal of Neuroscience. 29 (7), 1962-1976 (2009).
- Hwang, D. Y., Fleming, S. M., et al. 3,4-Dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: Behavioral characterization of a novel genetic model of Parkinson’s disease. Journal of Neuroscience. 25 (8), 2132-2137 (2005).
- Glajch, K. E., Fleming, S. M., Surmeier, D. J., Osten, P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson’s disease. Behavioural Brain Research. 230 (2), 309-316 (2012).
- Rockenstein, E., Mallory, M., et al. Differential neuropathological alterations in transgenic mice expressing a-synuclein from the platelet-derived growth factor and Thy-1 promoters. Journal of Neuroscience Research. 68 (5), 568-578 (2002).
- Fleming, S. M., Salcedo, J., et al. Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype a-synuclein. Neuroscienze. 142 (4), 1245-1253 (2006).
- Chen, L., Cagniard, B., et al. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. Journal of Biological Chemistry. 280 (22), 21418-21426 (2005).
- Pothakos, K., Kurz, M. J., Lau, Y. S. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neuroscience. 10 (6), (2009).
- Patki, G., Che, Y., Lau, Y. S. Mitochondrial dysfunction in the striatum of aged chronic mouse model of Parkinson’s disease. Frontiers in Aging Neuroscience. 1 (3), (2009).
- Rommelfanger, K. S., Edwards, G. L., Freeman, K. G., Liles, L. C., Miller, G. W., Weinshenker, D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proceedings of the national academy of sciences of the United States of America. 104 (34), 13804-13809 (2007).
- Li, Y., Liu, W., et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nature Neuroscience. 12 (7), 826-828 (2009).
- Crawley, J. N. . What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. , (2000).
- Crawley, J. N., Paylor, R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Hormones and Behavior. 31 (3), 197-211 (1997).
- Fernagut, P. O., Chalon, S., Diguet, E., Guilloteau, D., Tison, F., Jaber, M. Motor behaviour deficits and their histopathological and functional correlates in the nigrostriatal system of dopamine transporter knockout mice. Neuroscienze. 116 (4), 1123-1130 (2003).

