A High-throughput Method for Measurement of Glomerular Filtration Rate in Conscious Mice
Summary
Measurement of glomerular filtration rate (GFR) is the gold standard for kidney function assessment. Here we describe a high-throughput method which allows the determination of GFR in conscious mice by using a single bolus injection, determination of fluorescein isothiocyanate (FITC)-inulin in plasma and calculation of GFR by a two-phase exponential decay model.
Abstract
The measurement of glomerular filtration rate (GFR) is the gold standard in kidney function assessment. Currently, investigators determine GFR by measuring the level of the endogenous biomarker creatinine or exogenously applied radioactive labeled inulin (3H or 14C). Creatinine has the substantial drawback that proximal tubular secretion accounts for ~50% of total renal creatinine excretion and therefore creatinine is not a reliable GFR marker. Depending on the experiment performed, inulin clearance can be determined by an intravenous single bolus injection or continuous infusion (intravenous or osmotic minipump). Both approaches require the collection of plasma or plasma and urine, respectively. Other drawbacks of radioactive labeled inulin include usage of isotopes, time consuming surgical preparation of the animals, and the requirement of a terminal experiment. Here we describe a method which uses a single bolus injection of fluorescein isothiocyanate-(FITC) labeled inulin and the measurement of its fluorescence in 1-2 μl of diluted plasma. By applying a two-compartment model, with 8 blood collections per mouse, it is possible to measure GFR in up to 24 mice per day using a special work-flow protocol. This method only requires brief isoflurane anesthesia with all the blood samples being collected in a non-restrained and awake mouse. Another advantage is that it is possible to follow mice over a period of several months and treatments (i.e. doing paired experiments with dietary changes or drug applications). We hope that this technique of measuring GFR is useful to other investigators studying mouse kidney function and will replace less accurate methods of estimating kidney function, such as plasma creatinine and blood urea nitrogen.
Introduction
The rate of formation of plasma ultrafiltrate at the glomerulus has become the basis for evaluating renal function (glomerular filtration rate, GFR). The ideal marker to determine GFR should be freely filtered, neither secreted or reabsorbed and not metabolized. Such a behavior was found to be true for inulin, and inulin clearance has become a gold standard for determination of GFR 3. Whereas the clearance of creatinine is extensively used as a measure of GFR in humans and animals, creatinine does not fulfill the requirements of an ideal GFR marker. Around 10 – 40% of the creatinine clearance in humans is the consequence of active tubular secretion 1, 11, and renal secretion of creatinine is also prominent in mice and rats 4, 7, 9, 22. Renal dysfunction is known to increase the tubular secretion of creatinine which limits its value as a marker of GFR 1, 2. We have previously shown that organic anion transporter isoform 3 (OAT3) contributes to murine renal creatinine secretion 22. However, inulin clearance typically requires the use of radioactive labeled inulin (3H or 14C). The radioactive determination of inulin can be used in both anesthetized and conscious mice, but harbors the disadvantages of numerous safety regulations and strict handling issues inherent with the use of radioactive isotopes. Along those lines, surgical clearance preparations for determination of GFR are time consuming as well as terminal in nature. In 1999 Lorenz et al. 12 introduced FITC-inulin as a marker of single nephron GFR in anesthetized mice. Five years later Qi et al. 15 determined whole kidney GFR in conscious mice by using FITC-inulin. Alternative protocols are now using FITC-inulin as an exogenous marker to measure GFR in conscious and anesthetized mice. These protocols retain the sensitivity of radioactive inulin by using fluorescence detection and circumventing the disadvantages of working with a radioactive labeled marker. The FITC-inulin assay was modified by others 7, 8 and us 22, 23 to take advantage of the microvolume capability of the NanoDrop 3300 fluorospectrometer. This allows reducing the total sample volume needed to <100 μl blood per GFR measurement.
With the method and high throughput protocol provided in this publication, we demonstrate the feasibility of measuring GFR in up to 24 conscious mice per day. This technique could be useful to other investigators studying murine GFR and we hope it will replace less accurate methods as indices for kidney function, such as creatinine and blood urea nitrogen.
Protocol
1. Solutions
- 0.85% NaCl: 8.5 g NaCl/1 l ddH2O (8.5 g/L).
- 0.5 mol/L HEPES pH 7.4 (dissolve 59.6 g of HEPES in 0.5 L of ddH20 and adjust pH to 7.4 using 10 N NaOH).
2. Preparation of FITC-inulin Injection Solution
- Weigh FITC-inulin to prepare a 5% solution and dissolve in 0.85% NaCl (usually 100 mg/2 ml 0.85% NaCl) by heating to 90 °C until completely dissolved.
- Weigh dissolved FITC-inulin solution and note weight.
- Cut a 20 cm piece of dialysis membrane (molecular weight cut-off: 1,000) and put in ddH2O for 30 min to remove residual NaN3 from the membrane and flush a few times afterwards.
- Fill dissolved FITC-inulin into dialysis membrane and seal properly with closures.
- Weigh entire dialysis membrane.
- Put the dialysis membrane in 1 L 0.85% NaCl solution and stir slowly light-protected for 24 hr at room temperature.
- Weigh entire dialysis membrane again and calculate the new concentration (water will osmotically move into the membrane and unbound FITC, or bound FITC-inulin <1,000 molecular weight will move out of the membrane; thus, the concentration of FITC-inulin will decrease substantially).
- The new FITC-inulin concentration (c) is calculated using the following formula: c = n/V, where n = initial FITC-inulin amount, V = new volume (difference in weight of dialysis tubing before and after dialysis plus volume of initial FITC-Inulin solution).
- Prior to use sterilize dialyzed FITC-inulin solution by filtration through a 0.22 μm syringe filter.
- Keep FITC-inulin protected from light (aluminum foil) at 4 °C. Dialyzed and sterilized FITC-inulin can be used for up to 2 weeks. Participated FITC-inulin can be dissolved by re-heating at 90 °C for a few minutes.
Note: if using FITC-sinistrin 16-18, which does not require dialysis, all steps required for dialysis can be skipped.
3. Injection and Blood Collection
- Take body weight (bw) of the mice before the experiment.
- Anesthetize mice briefly with isoflurane.
- For high-throughput of 6 mice please follow protocol provided in Figure 3.
- Inject 2 μl/g bw of dialyzed FITC-inulin into the retroorbital plexus 24 by using a G30 ½ in needle (remove air bubbles from 100 μl Hamilton syringe by first using a G26 ½ in needle and then switching to a G30 ½ in needle for injection).
- Cut 1 mm of mouse tail with scissors once and collect blood at 3, 5, 7, 10, 15, 35, 56 and 75 min after injection in Na-Heparin minicapillaries. Seal minicapillaries after blood collection with Châ-seal.
- Keep samples protected from light.
- Put sealed minicapillaries inside hematocrit capillaries and centrifuge for 5 min.
- Break minicapillaries by using a diamond cutter and transfer entire plasma by pipetting into a 0.2 ml tube.
- Make a 1:10 dilution by using 2 μl of plasma and 18 μl 0.5 mol/L HEPES (pH 7.4) in new 0.2 ml tube (samples could be stored overnight at 4 °C for fluorescence measurements).
- Measure 2 μl of diluted sample with NanoDrop 3300 in duplicates. The description and usage of the instrument is described here 5.
4. Preparation of Standards
- Collect blood from mice of the same background strain in heparin coated hematocrit capillaries, spin down and dilute 1:10 with 0.5 mol/L HEPES (pH 7.4); usually 80 μl plasma + 720 μl HEPES.
- Dilute FITC-inulin injection solution with diluted mouse plasma to get the following standard dilutions:
1:10: 10 μl undiluted FITC-inulin solution + 90 μl diluted mouse plasma
1:100: 10 μl (1:10) solution + 90 μl diluted mouse plasma
1:1,000: 10 μl (1:100) solution + 90 μl diluted mouse plasma
1:2,000: 30 μl (1:1) solution + 30 μl diluted mouse plasma
1:10,000: 10 μl (1:5) solution + 40 μl diluted mouse plasma
1:20,000: 20 μl (1:1) solution + 20 μl diluted mouse plasma
1:100,000: 10 μl (1:5) solution + 40 μl diluted mouse plasma
The concentration of the standards will depend on the dialysis and will always be different for each preparation of FITC-inulin. It is necessary to enter the concentration for the standard curve new every time. - Analyze data to calculate GFR with appropriate software (e.g. GraphPad Prism) by using a two-phase exponential decay function as described in here 15, 20 and shown in Representative Results Section.
Representative Results
GFR will be calculated using the following formula:
GFR = n/(A/K1 + B/K2), where
n = injected amount (n = c•V, where c = FITC-inulin concentration, V = injected volume)
A = Span1, y-intercept of elimination
K1= decay constant for elimination
B = Span2, y-intercept of distribution
K2 = decay constant for distribution
Note: A, K1, B and K2 are abbreviations given by GraphPad Prism, every software capable of analyzing two-phase exponential decay can be used, however, other scientific software might use different abbreviations.
By employing a two-phase exponential decay curve fit, which is based on plasma FITC-inulin concentrations, a curve comparable to the one shown in Figure 1 should be obtained. Calculating GFR by the above shown formula (and as described in here 20) allows to detect impairment of kidney function 15 or glomerular hyperfiltration 8, 23 as found in mice with type 1 diabetes mellitus (Figure 2).

Figure 1. To measure GFR in conscious mice, plasma kinetics of FITC-inulin following a single-dose intravenous injection is used. For calculation of GFR, a two-compartment model is employed. In the two-compartment model, the initial rapid decay phase represents redistribution of FITC-inulin from the intravascular compartment to the extracellular fluid. The later phase, with slower decay in FITC-inulin concentration, predominantly reflects systemic clearance from the plasma.
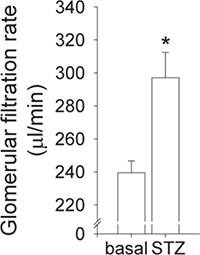
Figure 2. Detection of diabetic hyperfiltration in wild-type mice. Type I diabetes mellitus was induced by intraperitoneal application of streptozotocin (STZ, 50 mg/kg for 5 consecutive days). GFR was determined before (basal) and 5 weeks after induction of diabetes. Due to tubuloglomerular feedback, a primary increase in proximal reabsorption causes early diabetic hyperfiltration 21 a finding that is reproducible in wild-type mice (n=10, p<0.01 vs. basal same mouse).

Figure 3. Flow-chart for measurement of GFR in a set of 6 mice. To measure whole kidney GFR by the single bolus injection method, 8 blood samples must be collected at 3, 5, 7, 10, 15, 35, 56 and 75 min after FITC-inulin injection. Numbers indicate time points. Numbers in brackets indicate sample number. Time points left of the vertical red line indicate injection time points (at 0, 11, 22, 33, 44 and 55 min). Each mouse is marked with a different color. To get sequential blood collections follow the arrows. Grey boxes indicate when the next mouse must be prepared for isoflurane anesthesia prior to injection. Using this protocol the minimum time between 2 blood collections from different mice is 1 min.
Discussion
The present study describes a technique for determination of GFR in conscious mice by single-bolus injection and analysis of fluorescence in 8 blood samples by two-phase exponential decay. A GFR measurement in 1 mouse takes 75 min. By using a special flow-chart, it is possible to measure the GFR of 6 mice in 130 min. It is feasible to repeat this flow-chart protocol up to 4 times per day and measure GFR in 24 mice. We modified this method so that it is possible to collect blood from a small tail snip rather than tail vein puncture, which is quicker and easier for investigators to perform compared to tail vein puncture. By using 10 μl hematocrit capillaries and a 1:10 dilution, it is possible to reduce the total amount of blood needed to <100 μl. Finally, fluorescence is measured using a NanoDrop 3300 fluorospectrometer, which allows determination of fluorescence in 1-2 μl of diluted sample. This method will give the interested investigator a true GFR value. In contrast, creatinine measurements in mice have several weaknesses including “creatinine blind range” and difficulties related to specific and sensitive determination. The “creatinine blind range” limits its function as a marker for GFR because serum creatinine remains in the normal range until 50% of renal function is lost 19. Since several investigators utilize genetically modified mice in their research to study renal physiology and pathophysiology, this bears the following problem: assuming a knockout mouse of gene X would have a significantly lower GFR (by 40%) compared to a wild-type mouse, a simple screening by comparing plasma creatinine would not detect this difference. The need for a good accurate method of GFR determination becomes more critical the smaller the differences in GFR become.
Mice have high concentrations of non-creatinine chromagens in their plasma and urine that interfere with commercially available creatinine assays. As a consequence, assays which are based on the alkaline picrate Jaffé reaction overestimate creatinine concentration in plasma compared with high performance liquid chromatography (HPLC) determination 6, 13. Another limitation is that serum creatinine is below detection limits of several commercially available assays compared to HPLC determination. However, by using enzymatic reactions investigators were able to determine plasma creatinine in mice 10, 22.
GFR in a similar range were found in conscious mice using a comparable single-bolus injection method as well as urinary FITC-inulin clearance 14. In contrast to their protocol, our protocol using tail snip does not require special precautions to preserve the vessel integrity compared to tail vein or saphenous vein puncture. For the urinary/plasma based FITC-inulin clearance it is necessary to surgically implant two osmotic minipumps. Two pumps are necessary to achieve high enough steady-state plasma FITC-inulin concentrations which are clearly distinguishable from the background. This also requires the use of metabolic cages for a complete and timed urine collection. The cages need to be rinsed to account for a loss of 25-37 % of FITC-inulin, which otherwise occurs without rinsing.
The pitfalls of using this FITC-inulin method are limited. Investigators should be aware of the following: (i) assure that there are no air bubbles in the syringe used for injection of the FITC-solution, (ii) complete retrobulbar injection must be achieved because the amount injected determines the calculated GFR, and (iii) mice will most likely urinate during the 75 min GFR experiment, so avoid contaminating blood samples with urine, which will have a very high FITC-inulin concentration as a consequence of the kidneys concentration ability.
General advantages of this FITC-inulin method include the following: (i) it is a non-radioactive technique, (ii) it is possible to repeat measurements multiple times in the same mouse, (iii) the assay can be performed in conscious mice, (iv) no urine collection is required, and (v) a high-throughput option can be utilized. Disadvantages for the single bolus injection include the need for dialysis of FITC-inulin, which can be overcome by using a better FITC-marker, FITC-sinistrin, which is available now and can be measured transcutaneously using a special miniaturized device 18. Even though GFR is measured, it is not possible to measure fractional excretions of fluid or ions with this method.
Taken together, this method provides the feasibility of a high-throughput method for measuring GFR in conscious mice. Thus, this technique might be of interest for other investigators who are interested in determining renal function in mice.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
I thank Dr. Jessica Dominguez Rieg for critical reading of this manuscript. This work was supported by the American Heart Association (Scientist Development Grant 10SDG2610034), a Carl W. Gottschalk Research Grant of the American Society of Nephrology, the National Institute of Diabetes and Digestive and Kidney Diseases O’Brien Center for Acute Kidney Injury Research (P30DK079337), and the Department of Veterans Affairs.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| NaCl | Sigma-Aldrich | S7653 | |
| FITC-inulin | Sigma-Aldrich | F3272 | |
| HEPES | Sigma-Aldrich | H7706 | |
| Isoflurane | Webster Veterinary | 78366551 | |
| Dialysis membrane (MWCO 1000) | Spectrum Laboratories | 132636 | |
| Membrane closure | Spectrum Laboratories | 132736 | |
| 80 μl Hematocrit capillaries | Drummond Scientific | 22362566 | |
| Hematocrit centrifuge | IEC | Micro-MB | |
| 10 μl Na+-Heparin minicaps | Hirschmann | 9000210 | |
| Syringe (100 μl) | Hamilton | 80601 | |
| Fluorospectrometer | Thermo-Fisher Scientific | NanoDrop 3300 | |
| Filter (0.22 μm) | Spectrum Laboratories | 880-26464-UE | |
| Sealant | Châ-seal | 510 | |
| Needles (G26 ½ and G30 ½) | Becton Dickinson | 305111 & 305106 |
Riferimenti
- Bauer, J. H., Brooks, C. S., Burch, R. N. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am. J. Kidney Dis. 2 (3), 337-346 (1982).
- Carrie, B. J., Golbetz, H. V., Michaels, A. S., Myers, B. D. Creatinine: an inadequate filtration marker in glomerular diseases. Am. J. Med. 69 (2), 177-182 (1980).
- Chasis, H., Ranges, H. A., Goldring, W., Smith, H. W. The Control of Renal Blood Flow and Glomerular Filtration in Normal Man. J. Clin. Invest. 17 (5), 683-697 (1938).
- Darling, I. M., Morris, M. E. Evaluation of “true” creatinine clearance in rats reveals extensive renal secretion. Pharm. Res. 8 (10), 1318-1322 (1991).
- Dunn, S. R., Qi, Z., Bottinger, E. P., Breyer, M. D., Sharma, K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 65 (5), 1959-1967 (2004).
- Eisner, C., Faulhaber-Walter, R., et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 77 (6), 519-526 (2010).
- Faulhaber-Walter, R., Chen, L., et al. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J. Am. Soc. Nephrol. 19 (4), 722-730 (2008).
- FINGL, E. Tubular excretion of creatinine in the rat. Am. J. Physiol. 169 (2), 357-362 (1952).
- Keppler, A., Gretz, N., et al. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 71 (1), 74-78 (2007).
- Levey, A. S., Perrone, R. D., Madias, N. E. Serum creatinine and renal function. Annu. Rev. Med. 39, 465-490 (1988).
- Lorenz, J. N., Gruenstein, E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am. J. Physiol. 276, 172-177 (1999).
- Meyer, M. H., Meyer, R. A., Gray, R. W., Irwin, R. L. Picric acid methods greatly overestimate serum creatinine in mice: more accurate results with high-performance liquid chromatography. Anal. Biochem. 144 (1), 285-290 (1985).
- Qi, Z., Breyer, M. D. Measurement of glomerular filtration rate in conscious mice. Methods Mol. Biol. 466, 61-72 (2009).
- Qi, Z., Whitt, I., et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am. J. Physiol. Renal Physiol. 286 (3), F590-F596 (2004).
- Schock-Kusch, D., Sadick, M., et al. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol. Dial Transplant. 24 (10), 2997-3001 (2009).
- Schock-Kusch, D., Xie, Q., et al. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 79 (11), 1254-1258 (2011).
- Schreiber, A., Shulhevich, Y., et al. Transcutaneous measurement of renal function in conscious mice. Am. J. Physiol. Renal Physiol. 303 (5), F783-F788 (2012).
- Shemesh, O., Golbetz, H., Kriss, J. P., Myers, B. D. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 28 (5), 830-838 (1985).
- Sturgeon, C., Sam, A. D., Law, W. R. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparison of estimation analyses. J. Appl. Physiol. 84 (6), 2154-2162 (1998).
- Vallon, V., Blantz, R. C., Thomson, S. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: a tubulo-centric view. J. Am. Soc. Nephrol. 14 (2), 530-537 (2003).
- Vallon, V., Eraly, S. A., et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am. J. Physiol. Renal Physiol. 302 (10), F1293-F1299 (2012).
- Vallon, V., Schroth, J., et al. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron. Physiol. 111 (1), 30-38 (2009).
- Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M., Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Anim. (NY. 40 (5), 155-160 (2011).

