Tissue Triage and Freezing for Models of Skeletal Muscle Disease
Summary
The analysis of skeletal muscle tissues to determine structural, functional, and biochemical properties is greatly facilitated by appropriate preparation. This protocol describes appropriate methods to prepare skeletal muscle tissue for a broad range of phenotyping studies.
Abstract
Skeletal muscle is a unique tissue because of its structure and function, which requires specific protocols for tissue collection to obtain optimal results from functional, cellular, molecular, and pathological evaluations. Due to the subtlety of some pathological abnormalities seen in congenital muscle disorders and the potential for fixation to interfere with the recognition of these features, pathological evaluation of frozen muscle is preferable to fixed muscle when evaluating skeletal muscle for congenital muscle disease. Additionally, the potential to produce severe freezing artifacts in muscle requires specific precautions when freezing skeletal muscle for histological examination that are not commonly used when freezing other tissues. This manuscript describes a protocol for rapid freezing of skeletal muscle using isopentane (2-methylbutane) cooled with liquid nitrogen to preserve optimal skeletal muscle morphology. This procedure is also effective for freezing tissue intended for genetic or protein expression studies. Furthermore, we have integrated our freezing protocol into a broader procedure that also describes preferred methods for the short term triage of tissue for (1) single fiber functional studies and (2) myoblast cell culture, with a focus on the minimum effort necessary to collect tissue and transport it to specialized research or reference labs to complete these studies. Overall, this manuscript provides an outline of how fresh tissue can be effectively distributed for a variety of phenotypic studies and thereby provides standard operating procedures (SOPs) for pathological studies related to congenital muscle disease.
Introduction
Skeletal muscle is a unique tissue because of its structure and function, which can present a number of challenges when evaluating its pathology. While it is sufficient, and often even optimal, to evaluate most tissues following formalin fixation and paraffin embedding, this process leads to the production of cell shrinkage artifacts that impair the evaluation of critical phenotypes in congenital muscle disease (including myofiber size and shape). Additionally, frozen muscle is required when performing many histochemical stains (including the reduced nicotinamide adenine dinucleotide (NADH), cytochrome oxidase (COX), succinate dehyrogenase (SDH), adenosine triphosphatase (ATPase), and Oil Red O stains) that are essential for the detection of specific pathological abnormalities required to diagnose a variety of congenital myopathies, mitochondrial myopathies, and storage diseases. Many antibodies and molecular tools used to characterize muscle morphology and function have consequently been developed and optimized only for frozen tissue, which further limits the usefulness of fixed tissue preparations in studies of skeletal muscle biology. As a result, it has become standard practice to freeze skeletal muscle specimens for most clinical and research indications in the field of congenital muscle disease, despite the technical challenges that appropriate freezing might pose.
The freezing of skeletal muscle can be technically challenging due to the high likelihood of ice crystal formation within myofibers when an inappropriate freezing protocol is followed1. In most cases, freezing artifacts are encountered when the water content of the tissue is too high or when the freezing process is too slow. The water content of tissue can be accidentally increased by immersion in fluid, such as transport of the muscle in saline; or freezing can be compromised if the tissue has been entirely immersed in OCT. Both of these procedures can lead to catastrophic freezing artifacts in muscle tissue. Additionally, a number of small missteps in the freezing process may cause slow freezing or accidental thawing of samples after appropriate freezing, and these issues can similarly complicate pathological evaluation.
As new models and treatments for skeletal muscle disease are developed, it will become increasingly useful to use standard operating procedures (SOPs) to evaluate skeletal muscle phenotypes and treatment efficacy. This is particularly important for the pathological evaluation of congenital muscle diseases, as the recognition of subtle phenotypes seen in many of these disorders can be impaired by improper tissue preparation. This report represents an effort by a Congenital Muscle Disease Consortium to establish a skeletal muscle freezing SOP for the study of congenital muscle disease models.
The protocol presented here describes a strategy used in our clinical and research labs to optimize assessment of frozen muscle pathology, while also being suitable for a variety of protein-based and genetic studies. Specific strategies to prepare skeletal muscle specimens for electron microscopy are also presented. Additionally, as single fiber functional testing of frozen tissue is possible2,3, experts in these techniques have provided details on optimal tissue handling protocols. Unfortunately, the isolation of myoblast cell lines cannot be effectively performed on pre-frozen tissue, so a strategy is presented herein for the short-term storage and transport of muscle tissue for myoblast culture establishment at outside laboratories. Overall, the methods described here provide guidelines for the appropriate processing of muscle tissue for a variety of pathological, functional, molecular and cellular studies, regardless of the on-site capabilities of a given laboratory.
Protocol
Label the containers to be used for muscle storage with appropriate identifiers for the animal and muscle harvested. Pre-cool the bags/containers and instruments to prevent freeze/thawing of the tissue when it is added.
1. Study-design Considerations for Muscle Tissue Collection
- For small animal (murine) models of muscle disease, use an entire muscle for the study since small animal models often require evaluation of an entire muscle to provide sufficient myofiber sampling for pathological studies. For large animal (canine) models of muscle disease, use muscle portions that are at least 0.3 x 0.3 cm or greater in their transverse (cross-sectional) aspect.
NOTE: Core biopsies can also be used, but may be suboptimal for primary characterization studies due to sampling issues. - For studies involving the sarcomere, the ultrastructural measurement of sarcomere length may comprise a critical endpoint and may require specialized handling. This is usually only relevant in diseases where elements of the sarcomere show inappropriate lengths, as in some causes of nemaline myopathy. Some laboratories prefer to fix muscle in its non-contracted or stretched state, rather than performing these measurements on non-tensioned or non-stretched muscle.
- If sarcomere length will not be measured, fix the muscle tissue without pre-stretching the muscle by placing it directly into the desired EM fixative (see Reagents).
- If sarcomere lengths will be measured, several techniques to pre-stretch the muscle (see Discussion) may be used:
- Use a clamping device to hold the muscle at its resting tension while it is still in the animal, excise the muscle around the outside of the clamp, and place it directly into the desired EM fixative (see Reagents).
- Stretch and pin the muscle to a surface (such as a piece of cork) at a length similar to that observed in vivo after it has been extracted from the animal.
2. Sub-divide the Isolated Skeletal Muscle into Fragments that are Appropriate for the Desired Studies (Figure 1)
- For frozen muscle histology:
- Include as much of a given muscle as possible to minimize sampling bias (see step 1.1), but specimens should be <1.5 cm to avoid freezing artifacts.
- Treat all muscles from the same study similarly to prevent artifactual differences in fiber size due to differences in handling.
- For electron microscopy (EM):
- Cut a ~0.5 x 0.2 x 0.2 cm strip of muscle from the sample, with the longitudinal axis of the sample parallel to the longitudinal axis of the original muscle.
NOTE: The fixative will not adequately penetrate thicker pieces of tissue, so maintaining specimen thickness at 2 mm or less is crucial in obtaining appropriate results. Additionally, as orientation of such small fragments of muscle is often difficult, it is advisable to use a fragment of tissue that mimics the actual orientation of the muscle (i.e., the longest aspect of the fixed specimen is the longitudinal orientation of the muscle/myofibers) to minimize handling and confusion during EM processing.
- Cut a ~0.5 x 0.2 x 0.2 cm strip of muscle from the sample, with the longitudinal axis of the sample parallel to the longitudinal axis of the original muscle.
- For cell culture:
- The desired cell yield may affect the amount of muscle required for cell culture establishment. Single muscles (or portions of muscles) from small animals may provide insufficient cell numbers to establish cell cultures, so if necessary, pool tissue from multiple muscles.
3. Tissue Fixation and Processing for Electron Microscopy (EM)
- Place a fragment of muscle no greater than 2 mm in its thinnest dimension directly into the desired EM fixative (see Reagents).
- Place the muscle in EM fixative buffer (see Reagents) after 2-48 hr of fixation. Prolonged fixation (for weeks or months) can result in the loss of ultrastructural detail on EM studies.
- Send to an EM core facility for processing.
4. Muscle Freezing for Histological, Biochemical, and Molecular Studies
- Remove excessive moisture in the specimen by thoroughly blotting the specimen with a paper towel until the tissue is slightly adherent to the towel. Excessive moisture will produce significant freezing artifacts in the muscle.
NOTE: Tissue can be further dried by rolling it in baby powder. This step is generally not necessary for muscles unless they have been in an excessively moist environment. - Place OCT (or a similar adhesive such as gum tragacanth) in the bottom of a shallow cryomold or on cork, with only enough OCT to provide a foundation for the oriented muscle (Figure 2C). Labeling the cork or cryomold before freezing may be useful for specimen tracking.
- Carefully place the muscle into the mold in the desired orientation, with the majority of the muscle protruding from the OCT so that sectioned muscle is not in contact with OCT (Figure 2C).
- Add isopentane to a metal cup until it reaches a depth of approximately 3-4 cm.
- Put on thermal safety gloves and remove lid of Dewar.
- Place or hold the metal cup in contact with the liquid nitrogen, so that the level of liquid nitrogen on the outside of the cup is above the level of the isopentane on the inside of the cup. Strategies to arrange these containers are shown in Figure 2.
- Do not allow liquid nitrogen to enter the metal cup, as it produces a bubbling foam that can be cumbersome to work with (and is also insufficiently cold for freezing).
- Observe the isopentane to determine when it has reached the appropriate temperature. At the appropriate temperature (optimally between -140 to -149 °C), solid white pebbles of frozen isopentane will form (after a few minutes, and after the initial fog has disappeared above the isopentane) on the bottom of the cup, or the solution will generally thicken to the consistency of molasses. Freezing prior to this stage will yield freezing atifacts.
- If the isopentane entirely freezes solid, thaw and chill it to freezing temperatures again before subsequent use.
- Using pre-chilled forceps, lower the specimen and cork/mold into the isopentane for approximately 10-20 sec.
- Place the frozen specimen into a pre-cooled container.
- Keep specimen and container on dry ice at all times until transfer to a -80 °C freezer.
5. If Freezing Artifact is Present in Already Frozen Tissue, it is Possible to Improve the Degree of Freezing Artifact Using the Following “Thaw and Refreeze” Procedure
- Take blocks out of the freezer, one at a time, and keep them on dry ice. The timing of this protocol is critical. Only thaw and refreeze one block at a time.
- Move sample from dry ice to RT.
- If the sample was embedded in OCT, remove the surrounding OCT as completely as possible using a scalpel.
- Allow the sample to thaw at RT to the point where it is completely thawed, but not to the point where it is drying out.
- Gently prod the specimen with a forceps to ensure that the specimen is entirely thawed before progressing to the next step.
- Freeze the muscle as specified in steps 4.1-4.10.
6. Preparation of Muscle for Skinned Single Fiber Functional Testing
- The storage procedure for the muscle depends on when the muscle/fiber is being used for experiments.
- For use within a few weeks, place the muscle in a solution of 50% glycerol/50% relaxing solution (see Reagents), and store at -20 °C.
- For use after a few weeks, place the muscle in a solution of 75% glycerol/25% relaxing solution (see Reagents), and store at -80 °C.
- Alternatively, let muscle dry, pin to cork, and store on silica granules at -80 °C. These dehydrated muscles can be used for years.
7. Preparation of Fresh Muscle for Shipment of Cell Culture to Outside Laboratories
Protocols for establishment of cell cultures have been well-described elsewhere4-7.
- Fill a 50 ml sterile conical tube with sterile complete growth media.
- Add the muscle tissue to the tube using a sterile forceps, completely submerging the tissue in media.
- Fill the remainder of the tube with media to prevent drying during shipment.
- Add ice packs to the Styrofoam box and place the conical tube near several ice packs.
- Please note that only ice packs (and not dry ice) should be used to prevent damage from freezing of the liquid and tissue.
- Ship packages O/N, but tissues can last 2 days under these conditions.
Representative Results
To provide examples of the artifacts seen with improper freezing, several mouse quadriceps muscles were frozen using different techniques (Figure 3) to replicate commonly-encountered errors. As shown in Figure 3A, appropriately frozen skeletal muscle shows a tight apposition of myofibers to the surrounding tissue (usually other muscle fibers, but sometimes the endomysial space between myofibers is filled with fibrosis or inflammatory cells in a variety of disease or injury processes) and a clearly visible cytoplasmic compartment. For pathological analysis, the ability to view the intracellular space is often more important than the endomysial compartment, as it is usually critical to clearly identify the presence of degenerative changes, regenerative changes, or other pathognomonic findings (central nuclei, intracellular inclusions) in this space. Thus, the presence of ice crystals in the intracellular compartment represents a major problem in the pathological assessment of muscle.
Proper freezing of muscle tissue requires both sufficiently cold temperatures and a sufficient speed of freezing to avoid the formation of ice crystals. Even when accounting for both factors, considerable freezing artifact can still occur because of excessive moisture within the muscle tissue. This issue is most commonly encountered in muscle tissue that had been previously immersed in saline and insufficiently dried or when muscle has been in direct contact with OCT mounting media at the site of sectioning (Figure 3B, 3E). While some contact with OCT is usually unavoidable as a consequence of the mounting process, every effort should be made to avoid contact between areas that will be histologically evaluated and the OCT used for mounting. In contrast, samples frozen using a -80 °C freezer (Figure 3C) and liquid nitrogen (Figure 3D) provide examples of freezing artifact that is produced by suboptimal temperature or speed of freezing. The slow freezing process encountered by placing tissue in a -80 °C freezer provides an extreme example of freezing artifact. When using liquid nitrogen, the main problem is that contact between liquid nitrogen and the tissue causes a sheath of gaseous nitrogen to form at the tissue-liquid interface, and this gaseous interface is not sufficiently cold to produce rapid muscle freezing1. This can produce significant freezing artifact near the surface of the specimen, but internal areas of the specimen are more likely to flash-freeze at an appropriate rate and may be entirely spared of freezing artifact. While the simple immersion of muscle tissue in liquid nitrogen is nowhere near as useful as the isopentane-freezing process, it can be useful for the freezing of large muscle specimens in situations where isopentane is unavailable (such as clinical autopsy specimens at institutions without clinical muscle pathology laboratories). Results using this strategy are generally better when using large portions of tissue (>2 cm in several dimensions).
While the avoidance of freezing artifact provides optimal histological results, a technique has also been developed (and described herein) that substantially reverses freezing artifacts to allow the evaluation of improperly frozen tissue. Tissue that is improperly frozen (Figure 3E) can be thawed and refrozen under tightly controlled conditions (Figure 3F) to allow the redistribution of water within the fibers, and the degree of morphological preservation that is obtainable can be excellent. In our experience, this process preserves the intracellular morphology of the tissue to a remarkable extent, but it often produces an artifactual separation of fibers within the endomysial space. As many pathological endpoints are found in the intracellular space and those in the endomysial space are best viewed using special stains (either to highlight fibrosis or to identify other cell types), these artifactual changes are unlikely to truly impair the pathological evaluation of muscle.
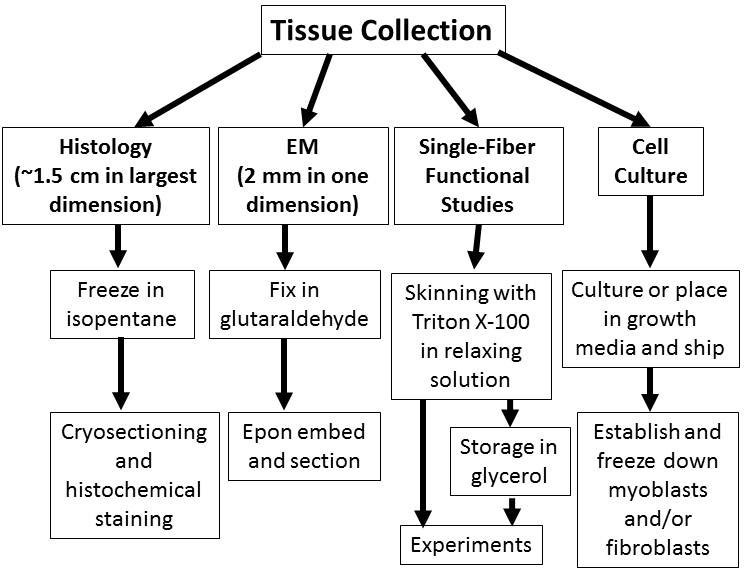
Figure 1. Triage of tissue for structural, functional, and cellular studies of skeletal muscle. Depending on the types of studies desired, collected skeletal muscle tissue can be processed in a variety of ways for optimal results. The protocols outlined in this paper describe methods for triaging or processing skeletal muscle specimens for off-site studies to optimize the results obtainable from expert core facilities.
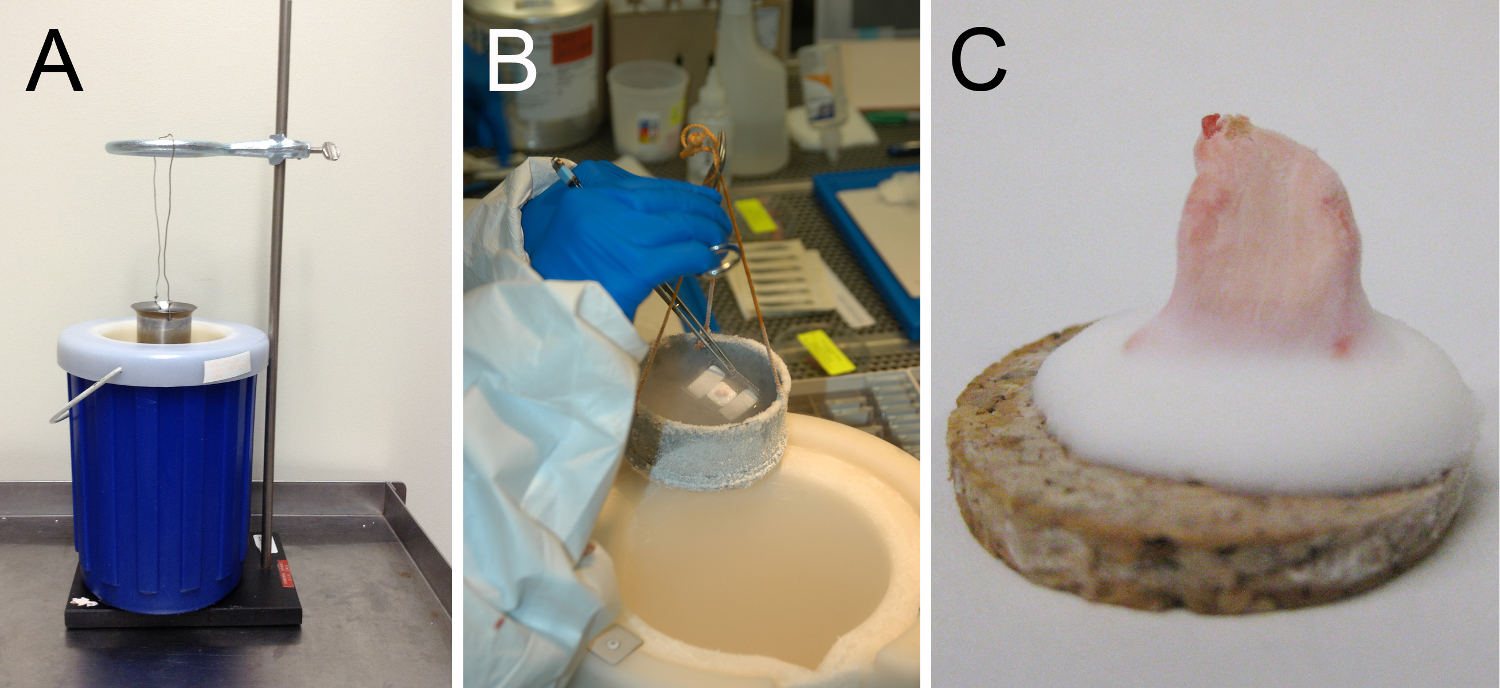
Figure 2. Examples of apparatuses used for muscle freezing. As described in the protocol, optimal muscle freezing requires immersion of muscle tissue in isopentane that is at its freezing temperature. A safe and efficient means of obtaining ice-cold isopentane involves the use of a metal container for the isopentane that is cooled in a surrounding Dewar containing liquid nitrogen. As it is often convenient to have both hands free, it is possible to use a brace such as a ring stand (A) to hold the isopentane container in the liquid nitrogen while preventing the mixture of the two liquids. Alternatively, the container could also be held in the liquid nitrogen manually (B). In either case, the tissue should not be placed into the isopentane until the isopentane is visibly freezing at the bottom of the container (usually as opaque white pebbles), and then should be immersed for 10-20 sec to allow complete freezing. Isopentane at freezing temperature produces minimal “fog”, (i.e., the temperature is inadequate if considerable fog is present). Once the tissue is frozen, place it directly into pre-cooled containers (and then directly into a cooler or freezer) to prevent accidental thawing after freezing. (C) Appropriately-mounted frozen muscle should have the majority of the muscle tissue protruding from a base of frozen OCT.

Figure 3. Examples of freezing artifact, and of effective thawing/refreezing. Representative images of (A) appropriately isopentane-frozen muscle, (B) muscle frozen appropriately in isopentane, but while in contact with OCT mounting medium, (C) muscle frozen in a -80 °C freezer, and (D) muscle frozen in liquid nitrogen, all of which display varying degrees of freezing artifact. As shown in (B), the high liquid content of OCT produces significant freezing artifact in muscle that is in contact with it, so only the minimal amount of OCT required for mounting should be used, and the muscle for histological evaluation should be “standing out” from its surface. The slowness of the freezing process using a (C) -80 °C freezer or (D) liquid nitrogen (relative to the speed of freezing in ice cold isopentane) can lead to the production of significant freezing artifact. Panels E and F show muscle from the same specimen that was (E) initially suboptimally frozen (due to excessive contact with OCT) and then (F) thawed and refrozen using the technique described herein. While one can observe some artifactual separation between fibers in the refrozen sample, the improvement in the intracellular morphology of the muscle fibers is readily apparent. Please click here to view a larger version of this figure.
Discussion
Skeletal muscle is a structurally and functionally unique tissue, and specialized preparation procedures are necessary to allow the optimal assessment of structural and functional parameters. While a variety of tissues are commonly frozen for pathological studies in clinical and research contexts, freezing protocols for non-muscle tissues usually involve the total immersion of the tissue in OCT before freezing. As shown in Figure 3, such a protocol is unsuitable for the pathological evaluation of skeletal muscle and yet is similar enough to the protocol described here that this is a commonly encountered error. The goal of this paper is to provide a straightforward protocol for the proper handling of muscle to avoid issues like this. Advice has also been compiled on the proper handling of muscle for off-site physiological and cellular studies in an effort to facilitate the acquisition of high quality data by outside core laboratories in cases where on-site studies are not preferable or possible.
As noted in this protocol, elements that are absolutely essential in the proper processing of muscle for pathological studies include minimizing the water content of the tissue, decreasing the temperature at which the muscle is frozen, and increasing the speed at which freezing is achieved. Excessive moisture within the tissue or excessive slowness of the freezing process (produced by insufficient temperatures or by lack of direct contact between the freezing agent and the tissue, as is encountered with liquid nitrogen) will lead to freezing artifacts that can impair pathological analysis. As OCT provides an additional source of tissue moisture, many laboratories use other adhesives like gum tragacanth as an embedding substrate. Even in cases where freezing is performed appropriately, care should be taken to avoid subsequently accidentally thawing specimens through contact with RT containers or instruments. Thus, a successful freezing process requires a degree of planning that includes a pre-chilling of all instruments and containers to be used. When freezing artifacts are encountered, there is a method described here for the recovery of the tissue that is sufficient for most pathological evaluations. However, this freeze/thaw cycle does not offer perfect histology and has the potential to impair other molecular or enzymatic studies of the tissue (in addition to the time required to re-freeze the tissue), so using appropriate initial freezing practices is vastly preferable. In cases where freezing artifact is encountered on pre-frozen tissue, however, the technique described herein can be extremely useful.
Fixation and processing of tissue for EM can offer specific technical challenges that require planning prior to tissue collection. The most common error when collecting specimens for EM involves the use of tissue fragments that are too thick for glutaraldehyde to penetrate. As glutaraldehyde only penetrates approximately 0.1 cm into muscle tissue from a given surface, care should be taken to ensure that one dimension of the EM samples is no thicker than 0.2 cm. Additionally, as EM is an excellent means of directly evaluating the contractile apparatus, some investigators have developed strategies to pre-tension or pre-stretch the muscle prior to fixation to allow the measurement of contractile elements at a physiologically relevant tension. There is no standard protocol for pre-tensioning, but two strategies are briefly described in this protocol. It should be noted that attempts to pre-tensioning the muscle can produce unpredictable results unless they are done in a very specific manner, and it may be preferable to fix muscles in the slack state to prevent artifactual changes in sarcomere length through a non-standard pre-tensioning procedure8,9. For muscles in which such specific measurements are not necessary (including most biopsies performed for clinical purposes), efforts to pre-tension the muscle are generally not made, and the main effect on muscle morphology is a non-uniform spacing of sarcomeres in the muscle.
This paper represents the first in a series to provide SOPs for the performance of tests in the congenital muscle disease field, and it represents the efforts of over 20 experts in the congenital muscle disease field that routinely perform cellular, molecular, functional, physiological, and pathological research. A range of published SOPs will be made available over the coming year, and the necessary protocols and appropriate publication formats for each were discussed at a Congenital Muscle Disease Consortium Workshop held in April of 2013 in Washington D.C. The goal of this SOP effort is to provide a roadmap for the necessary testing and analysis of specimens in the congenital muscle disease field to 1) standardize the practices and endpoints used in our field as much as possible, and 2) provide instruction on standard practices for new researchers in our field. We believe that these resources will facilitate the entry of new investigators into our under-studied field and thus improve the scope of research that can be performed. Additionally, a standardization of practices will be extremely helpful to compare data across studies and to identify endpoints when planning and performing preclinical and clinical trials.
While the main focus of this article is related to the appropriate freezing and preparation of tissue for a variety of studies, our collaborative group also discussed the useful endpoints for the pathological analysis of muscle specimens. At present, there is no official consensus on the approach to take when performing pathological analysis, and a variety of different studies are performed to relate new findings to prior publications for each respective disease. Thus, we thought that it would be useful to propose some general guidelines for the planning of pathological endpoints in muscle pathology characterization. Prior to quantifying the pathology in a study, considerable thought should be put into 1) the method of fiber size measurement, 2) the possibility of fiber-type-specific abnormalities or treatment effects, 3) the possibility of abnormalities or effects that are restricted to individual muscles, and 4) a strategy for quantifying pathological findings that are characteristic for the disease under study. Fiber size is a necessary endpoint for most studies, and unfortunately there is extensive variation in how it is quantified. Many studies report these results using automated quantification methods provided by proprietary imaging software, but many of these programs take shortcuts (such as assuming that the fibers are circles or ellipses) that can render these automated measurements inaccurate. It is necessary to understand how these automated programs make their measurements before having confidence in the measurements, and we encourage investigators to include these details in paper methods. Additionally, the specific measurement used to denote fiber size is extremely variable, and some measurements are preferable to others10,11. A commonly used measurement of fiber size is the fiber cross-sectional area (CSA), particularly because investigators performing physiological studies standardize their results to CSA measurements obtained using their instruments. Unfortunately, while CSA measurements can accurately reflect fiber size in perfect transverse sections, they are extensively dependent on fiber orientation (to the extent that longitudinal or obliquely-sectioned fibers will have artificially high CSA measurements) and are thus not ideal measurements for fiber size. A preferable measurement of fiber size that is less dependent on fiber cross-sectional area is the minimum Feret’s diameter (MinFeret diameter), which is the measurement of the minor diameter in the muscle cell12. This measurement is only slightly dependent on fiber orientation and is generally the clinical gold-standard for fiber measurement, and investigators are encouraged to move toward the use of this technique. These measurements can often be made using the same software that generates CSA measurements13, and are also straightforward to measure manually. With respect to evaluating the pathological data according to fiber type, specific muscle, and in the context of pathological findings related to the specific disease, these are less controversial issues that should just be considered during the planning of a study. Fiber type can be evaluated using immunohistochemical or ATPase staining, but it is useful to consider that specific muscles and animal species have specific mixtures of these fiber types (thus necessitating different expectations and testing strategies). Muscle specific pathological involvement or treatment efficacy can occur, and total muscle weight compared to controls can be used to identify the degree of heterogeneity of disease before deciding on the muscles to pathologically evaluate. Finally, it is well known that many muscle diseases are associated with specific pathological abnormalities (such as nemaline rods in nemaline myopathy)14,15, and so it is also useful to consider whether these abnormalities are found in a fiber-type- or muscle-specific distribution when performing the analysis16,17. Overall, while we do not propose an inflexible set of standards for the evaluation of muscle, we do believe that these issues should be considered prior to the performance of pathological studies in any skeletal muscle disease.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This publication is funded through Cure CMD, an Association Contre Les Myopathies (AFM) grant (project 16297), and the National Institutes of Health (grant numbers K08 AR059750 and L40 AR057721). We would also like to thank Dr. Julie Tetzlaff for her assistance in revising this manuscript.
Materials
| For tissue freezing | |||
| Liquid nitrogen Dewar with a liquid withdrawal device | Custom Biogenic Systems | Lab-5 Series | Any other liquid nitrogen dewar could substitute. |
| Cold-conductive container | Fisher | 02-583A | |
| Wide insulated container capable of holding liquid nitrogen | Nalgene | 4150-2000 | |
| Oakton Temp 10 Thermocouple Thermometer | Thomas Scientific | 1228Y01 | Optional, but can be very useful in identifying the point at which isopentane is freezing. |
| For single fiber functional testing | |||
| Petri dish | Fisher Scientific | 08-757-11 | |
| Sylgard coating | Ellsworth Adhesives | 3-6636 | |
| Dissection stereomicroscope | Harvard Apparatus | 693101 | Any other dissection microscope could substitute. |
| For cell culture | |||
| Sterile laminar flow hood | Thermo Scientific | 51022485 | Any other cell culture hood could substitute. |
| Materials | |||
| For tissue freezing | |||
| Plastic bags/containers | Nasco | B01009WA | |
| Thermal safety gloves | Denville | G4162 | |
| Face shield | Fisher Scientific | S47640 | |
| Long forceps | Fisher Scientific | 12-460-807 | |
| Marker | Staples | 125328 | |
| For cell culture | |||
| Sterile 50 mL conical tubes | Denville | C1060-P | |
| PES filter unit, 500 mL, 0.22 µm | Denville | F5227 | |
| Sterile forceps | Fisher Scientific | 644320 | |
| Ice packs | Fisher Scientific | NC9909223 | |
| Insulated Styrofoam box | Polar Tech | 207F | |
| Packing tape | Staples | 795570 | |
| Reagents | |||
| Tissue Freezing | |||
| Liquid nitrogen | Airgas | NI NF160LT350 | Store liquid nitrogen in containers designed for low-temperature liquids |
| Isopentane (2-methylbutane) | Sigma | M32631-4L | Store Isopentane in a flammable materials cabinet. |
| EM fixative (choose one) | The buffers without fixative should also be available, as EM-fixed tissues should be transferred from fixative to buffer after several hours or days if they are not immediately processed for EM. | ||
| 2.5% glutaraldehyde in 0.1M cacodylate buffer | Electron Microscopy Sciences | 16537-15 | |
| Karnovsky's fixative (3% glutaraldehyde + 2% paraformaldehyde in 0.1M phosphate buffer) | Electron Microscopy Sciences | 15732-10 | |
| For functional studies (if desired) | |||
| Relaxing solution, containing (in mmol/L) | |||
| 40 BES | Sigma | B9879 | |
| 10 EGTA | Sigma | E4378 | |
| 6.56 MgCl2 | Sigma | M8266 | |
| 5.88 Na-ATP | Sigma | A3377 | |
| 1.0 DTT | RPI | D11000 | |
| 46.35 K-propionate | Make a solution by mixing proprionic acid (Sigma P1386) and KOH (Fisher P250) 1:1 | ||
| 15 creatine phosphate, pH 7.0 | Sigma | P7936 | |
| 0.4 leupeptin | Calbiochem | 10895 | |
| 0.1 PMSF | Sigma | P7676 | |
| Skinning solution, containing | |||
| Relaxing solution (See Above) | |||
| 0.5% Triton X-100 | Sigma | T8787 | |
| Indicating silica granules | |||
| Flat pieces of cork (1 x 1 inch) | Electron Microscopy Sciences | 63305 | |
| Glycerol | Sigma | G2025 | |
| For cell culture (if desired) | |||
| Complete Growth Medium, containing (for 500 mL) | Sterilize by filtering the solution through a 500 mL 0.22 µm PES filter unit. Store at 4°C and use within 1 month. | ||
| 395 mL of high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) | Sigma | D5671 | |
| 100 mL of fetal bovine serum (FBS) | Sigma | F6178 | |
| 5 mL of 100X Penicillin-Streptomycin-Glutamine (PSG) | Sigma | G1146 | |
| Storage requriements | |||
| See the materials safety data sheet (MSDS) for all other reagents for storage specifications | |||
Riferimenti
- Dubowitz, V., Sewry, C. . Muscle Biopsy: A Practical Approach. 15, 407-442 (2007).
- Lawlor, M. W., et al. Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Hum Mol Genet. 22, 1525-1538 (2013).
- Talmadge, R. J., Roy, R. R. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 75, 2337-2340 (1993).
- Blau, H. M., et al. Differentiation properties of pure populations of human dystrophic muscle cells. Exp Cell Res. 144, 495-503 (1983).
- Lawlor, M. W., et al. Myotubularin-deficient myoblasts display increased apoptosis, delayed proliferation, and poor cell engraftment. Am J Pathol. 181, 961-968 (2012).
- Pavlath, G. K., Gussoni, E. Human myoblasts and muscle-derived SP cells. Methods Mol Med. 107, 97-110 (2005).
- Webster, C., Blau, H. M. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 16, 557-565 (1990).
- Ottenheijm, C. A., et al. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum Mol Genet. 18, 2359-2369 (2009).
- Witt, C. C., et al. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 25, 3843-3855 (2006).
- Garton, F., et al. Validation of an automated computational method for skeletal muscle fibre morphometry analysis. Neuromuscul Disord. 20, 540-547 (2010).
- Kim, Y. J., et al. Fully automated segmentation and morphometrical analysis of muscle fiber images. Cytometry A. 71, 8-15 (2007).
- Lawlor, M. W., et al. Inhibition of activin receptor type IIb increases strength and lifespan in myotubularin-deficient mice. Am J Pathol. 178, 784-793 (2011).
- Mula, J., et al. Automated image analysis of skeletal muscle fiber cross-sectional area. J Appl Physiol. 114, 148-155 (2013).
- Dubowitz, V., Sewry, C. . Muscle Biopsy: A Practical Approach. , 407-442 (2007).
- Nance, J. R., et al. Congenital myopathies: an update. Curr Neurol Neurosci Rep. 12, 165-174 (2012).
- Clarke, N. F., et al. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol. 63, 329-337 (2008).
- Lawlor, M. W., et al. Mutations of tropomyosin 3 (TPM3) are common and associated with type 1 myofiber hypotrophy in congenital fiber type disproportion. Hum Mutat. 31, 176-183 (2010).

