Protocol for Studying Extinction of Conditioned Fear in Naturally Cycling Female Rats
Summary
Gonadal hormones such as estrogen modulate memory formation in a number of experimental paradigms including fear extinction memory. This protocol describes a set of methods for investigating the influence of gonadal hormones specifically during extinction in naturally cycling females, including estrous cycle monitoring and exogenous estrogen administration.
Abstract
Extinction of conditioned fear has been extensively studied in male rodents. Recently, there have been an increasing number of studies indicating that neural mechanisms for certain behavioral tasks and response behaviors are different in females and males. Using females in research studies can represent a challenge because of the variation of gonadal hormones during their estrous cycle. This protocol describes well-established procedures that are useful in investigating the role of estrogen in fear extinction memory consolidation in female rats. Phase of the estrous cycle and exogenous estrogen administration prior to extinction training can influence extinction recall 24 hr later. The vaginal swabbing technique for estrous phase identification described here aids the examination and manipulation of naturally cycling gonadal hormones. The use of this basic rodent model may further delineate the mechanisms by which estrogen can modulate fear extinction memory in females.
Introduction
Inherent sex differences are observed across various cognitive behaviors and learning paradigms in both humans and rodents. For example, it has been reported that women generally have stronger verbal and detail-oriented abilities, whereas men have better spatial abilities1-3. These sex differences may be due in part to the influence of gonadal hormones. High estradiol levels improve performance on tasks in which women are better, but worsen performance on tasks that men typically do well4-6. Although this evidence is compelling, the limited ability to completely control experimental environments in human studies makes it difficult to determine if these effects on behavior can be attributed specifically to hormones. Animal studies, in contrast, allow for fully controlled circumstances.
Although the basic neural mechanisms of fear extinction have been identified and are well studied in males, it is unclear whether these systems are the same in females or how they change across the estrous cycle. Fear conditioning and extinction are widely used behavioral paradigms in rodents and humans to conduct studies related to anxiety disorders. Given that women have a higher risk for anxiety disorders as well as higher symptom duration and severity7-13, it is critical to include females in these studies. The underrepresentation of females in this research may be attributed to the challenges of estrous phase monitoring and accounting for the behavioral effects of sex hormones. Laboratories that do examine females in this regard encounter issues that often go unreported or are not described in the methods for their studies.
Emerging evidence in rodents suggests that sex differences in fear extinction are modulated by gonadal hormones14-18. In fear conditioning, animals are trained to fear a particular stimulus. After a number of unreinforced presentations of the stimulus, the animals learn not to fear the cue, a process called extinction. How well the animal learns and consolidates the memory of learning this task can be observed during an extinction recall test given some time after the extinction training. Less fear expression during extinction recall demonstrates good extinction memory consolidation. Recent findings from our laboratory suggest that estrogen can modulate the consolidation of fear extinction memory and improve extinction recall. Specifically, female rats that are extinguished in proestrus, the phase of the estrous cycle during which circulating estrogen levels peak, exhibit enhanced retention of extinction memory. In contrast, females that are extinguished in the low-estrogen metestrus phase display relatively poor extinction recall, which can be improved with exogenous estradiol administration prior to or immediately after extinction training15,16. The neural mechanisms of extinction memory consolidation (including the role of gonadal hormones) in females are not clear.
In laboratory animals, the role of hormones can be investigated using invasive surgical removal procedures such as castration and ovariectomy. Subsequent to recovery from surgery, gonadal hormones are often exogenously manipulated during the performance of a behavioral task19. This approach has provided critical information about sex hormones and is useful because it allows for well-controlled manipulations of gonadal hormones (in terms of timing and dose)20-23. This approach, however, does not assess the influence of the naturally occurring fluctuations that occur across the estrous cycle nor do they represent “normal” animals, thereby limiting the translational potential to human studies. It has been well-documented that female sex hormone levels peak and decline at specific phases of the estrous cycle, and estrogen receptor expression changes within the cycle, and after ovariectomy24. Thus, there is a need to conduct studies on females with intact gonads and refine experimental designs to reliably study the effects that high and low estrogen states may have on females throughout their lifespan.
This protocol focuses on the effect of estrogen on the neurobiological systems involved in fear extinction. It describes how to: 1) carefully monitor the estrous cycle, 2) prepare effective doses of estradiol for systemic administration, and 3) follow a behavioral paradigm that includes fear conditioning, extinction, and recall in naturally cycling female rats. This protocol can be modified with other pharmacological manipulations and cellular and molecular tools to aid studies to better understand sex differences that are observed in conditioned fear extinction behavior. Note that the procedures described below are those utilized within our laboratory, and there exist a number of variations of these procedures in the literature.
Protocol
NOTE: All procedures in this protocol have been approved by the Subcommittee on Research Animal Care, which serves as the Institutional Animal Care and Use Committee (IACUC) for the Massachusetts General Hospital, and are in compliance with the National Institutes of Health guidelines.
1. Animal Housing and Handling Procedure
- Upon arrival, house adult Sprague Dawley female rats about 8-10 weeks of age (weighing 200-225 g) in groups of 3-4 inch plastic bins with woodchip bedding. Maintain them on a diet of ad libitum access to chow and water and a 12 hr light/dark cycle. Leave rats to acclimate to the housing conditions and animal facility for a minimum of 1 week.
NOTE: All procedures in this manuscript are conducted during the light cycle. - Following this acclimation period, handle all animals for 5 min each for 2-3 days. Properly pick up the rats one at a time (supported by the torso and not by the tail), hold them securely, and pet them during this time. Handle animals that appear more stressed, jumpy, or anxious for longer periods of time. Do this to minimize handling stress, which could affect the following steps in this protocol.
NOTE: The number of days and amount of time handling the animals can vary between different labs; however, these should be consistent between experiments within the same lab.
2. Monitoring the Estrous Cycle: Vaginal Swab Smearing and Staining Procedure
- Make 0.9% saline solution (sodium chloride dissolved in distilled water). Moisten a cotton-tipped applicator in this solution. Blot the tip using a paper towel to avoid oversaturation, which could lead to inadequate sample collection or loss of cells. Holding the tail upward away from the vaginal opening, gently insert the cotton tip into the vaginal canal and roll around the walls to capture loose vaginal cells.
- If the animal urinates on the cotton tip, replace with a new one, as urine contamination will make it difficult for phase identification. Be extremely careful as to not cause distress to the animal during this procedure because exposure to stress will disrupt the estrous cycle. When swabbing, aim to achieve a swift insertion and withdrawal that is not too deep as to minimize extra stimulation to the cervix, which can induce pseudopregnancy.
NOTE: Pseudopregnancy can be identified as an acyclic estrous cycle that typically lasts for about 12 days, i.e. consecutive days of diestrus25. - Carefully remove the applicator tip and roll it onto a pre-labeled microscope slide. Avoid pressing down too hard as too much fluid on the slide can make phase identification difficult once dry.
NOTE: One slide can contain multiple days for each animal, although this can be done according to individual preferences. - Make sure the sample transfers onto the slide. Collect samples for several consecutive days on one slide to be able to better track and identify daily estrous phases changes.
NOTE: If possible, it is also ideal if the people collecting samples remain the same for all animals. - Once the slide is dry, begin the staining protocol for phase identification. Using the staining kit (see Table of Materials), immerse slides 10 times in the fixative (light blue), 10 times in stain solution (pink), and 5 times in counterstain solution (purple). Rinse slides gently under running water. Let slides completely air dry before viewing under microscope.
NOTE: The typically 4-5 day long estrous cycle consists of (in this order): estrus (E), metestrus (M), diestrus (D), and proestrus (P)26. Estrus is characterized by blue, cornified cells, metestrus by leukocytes and a combination of cornified and nucleated cells, diestrus is similar to metestrus in presence of cell types except they are very sparse, and proestrus by purple-stained aggregates of nucleated cells (Figure 1). There are various staining procedures, in addition to those described above, that are equally effective for vaginal smear cytology. - Collect samples for at least two complete cycles (~8-10 days) in order to monitor the phases accurately prior to experimentation. Be consistent with the time of day the samples are taken. Collect vaginal smear samples daily throughout and until the end of the experiment.
3. Pre-exposure
- Prior to the initiation of the experiment, pre-expose the animals to the conditioning chambers allow the animals to acclimate to the context (conditioning chambers) before any behavioral testing.
- Pre-expose the females to the conditioning chambers (located in sound-attenuating boxes) with the house lights on for 20-30 min per day for 3 days prior to any experimentation. Assign the box they are pre-exposed to as their chamber for the study. Thoroughly clean the boxes (walls and trays) between sessions and animals to remove odors since they can affect behavior and estrous cycle.
4. Day 1: Habituation/Fear Conditioning
- Once the animals have been verified to be in the estrus phase of their estrous cycles, begin the habituation/conditioning phase of the 3-day experiment.
- Take pre-tone baseline measures of freezing prior to the first tone conditioned stimulus(CS) presentations on each day of the experiment. Do this by acquiring percent freezing scores(calculated by dividing the seconds spent immobile by the duration of trial, multiplied by 100) during the stimulus-absent time interval at the beginning of the first CS-alone trial before the CS onset.
NOTE: These measures can be used to assess and compare baseline freezing levels with those during habituation, conditioning, extinction, and recall. - To conduct extinction training during the metestrus phase, condition the rats in their last day of estrus. Swab and identify estrous phase (step 2) each day before starting the experimental protocol (conditioning/extinction/recall). As the estrus phase may last longer than one day, use cycle history to predict length of phases to determine when the animals are ready to begin training.
- Take pre-tone baseline measures of freezing prior to the first tone conditioned stimulus(CS) presentations on each day of the experiment. Do this by acquiring percent freezing scores(calculated by dividing the seconds spent immobile by the duration of trial, multiplied by 100) during the stimulus-absent time interval at the beginning of the first CS-alone trial before the CS onset.
- In the conditioning chambers, connect the foot-shock grid floor to an electric stimulation generator. Use a stimulation level of 0.5-0.6 mA as the unconditioned stimulus (US). Use a 4 kHz, 80 dB tone as the conditioned stimulus (CS).
NOTE: These parameters may vary across laboratories and may be modified for optimal conditioning.- If the freezing analysis program requires visual cues (as opposed to temporal), co-activate an LED light with the tone. Position the light on the outside of the operant chamber, within the video frame. At the start of each trial, activate the LED light and keep it illuminated until the end of the trial.
NOTE: This light is not visible by the animal and only serves as a marker for the initiation and end of the trial within the recorded video files (to be used to measure freezing behavior).
- If the freezing analysis program requires visual cues (as opposed to temporal), co-activate an LED light with the tone. Position the light on the outside of the operant chamber, within the video frame. At the start of each trial, activate the LED light and keep it illuminated until the end of the trial.
- Place the animals into the conditioning chambers for 5 CS-alone trials of habituation. Immediately following the habituation session, perform 7 paired CS-US trials of fear conditioning. Ensure that the CS tone lasts for 30 sec and coterminates with the 0.5 sec US shock. Run trials in all sessions (habituation, conditioning, extinction, and recall) with a variable intertrial interval averaging 3 minutes.
- At the end of the conditioning session, return the animals to their home cages and to the animal facility until the next day.
5. Day 2: Estradiol Preparation
- Swab and identify the estrous phase for all animals that underwent Day 1 of this protocol. Prepare estradiol (also known as E2, 17beta-estradiol, or beta-estradiol) for a 15 μg/kg dose subcutaneous injection using sesame oil or 0.9% saline as vehicle.
- To calculate the amount of estradiol to prepare for injections, use the body weights of all animals, which were measured and recorded when they were conditioned on Day 1 of this protocol.
- Make calculations to standardize the administered volume of estradiol (i.e. 0.2 ml) per rat. Since estradiol is difficult to dissolve due to its water insolubility and requires a large volume of solvent for the small dose, prepare a stock solution by mixing estradiol in sesame oil over heat until dissolved. The solution can then be passed through a 0.22 μm filter to remove contaminants. For example, add a calculated volume of a 3,000 μg estradiol/1 ml sesame oil stock solution to the total needed volume of sesame oil in order to achieve a 15 μg/kg dose in a 0.2 ml injection volume per animal.
NOTE: Alternatively, use other forms of estrogen that are water-soluble and easier to dissolve.
6. Day 2: Estradiol Administration/Extinction Training
- Administer the estradiol subcutaneously. Lift the loose skin between the scapula by the neck in a gentle pinch and insert the syringe needle into the triangle that is formed by the skin folds.
- 30 minutes following subcutaneous injections of estradiol, place the animals in the conditioning chambers again and begin extinction training. In this extinction session, perform 20 CS-alone trials, with each trial consisting of a 30 sec, 4 kHz, 80 dB tone, presented at a variable intertrial interval averaging 3 min.
NOTE: The effect of estradiol on recall occurs when estradiol is administered prior to extinction training or immediately after extinction. The effect is not observed 4 hr post-extinction, so it is recommended to inject close in time to the beginning or end of the training session15.
7. Day 3: Extinction Recall
- 24 hr after the extinction training, swab the animals that completed Day 1 and 2 of this protocol.
- Place the animals into their assigned conditioning chambers (same as Day 1 and 2). Begin the extinction recall session by presenting 3 CS-alone trials (the three trials of extinction recall), which consists of only 3.80 dB CS-alone trials with intertrial intervals similar to the 20 trials administered for extinction training as described in step 6.2.
- Upon completion of the recall session, return the animals to their home cages.
8. Data Analysis
- Acquire behavioral data through video and analyze them using computer software.
NOTE: Freezing scores can also be manually counted by an experimenter that is blind to the drug treatment by timing freezing bouts during the CS presentation of each trial, expressed as percentage of the time spent freezing during the tone. Freezing is defined by complete lack of movement with the exception of breathing during the 30 sec CS-US trials. Calculate percent freezing by dividing the seconds spent freezing (immobile) by 30 seconds (duration of trial) multiplied by 100.
Representative Results
In this fear extinction recall protocol, percent freezing was measured as an indicator of fear. Animals that extinguished well and retained the memory of the extinction training exhibited low fear on the last day of the behavioral testing during extinction recall. Male and female rats do not significantly differ in conditioned fear expression during the conditioning, extinction, and recall phases (Figure 2). However, a sex difference becomes evident when the animals are analyzed separately as high- and low-estrogen estrous phase groups. Female rats that undergo extinction training during the low-estrogen metestrus phase of the estrous cycle do not recall the extinction memory on day 3 as well as those trained in proestrus (Figure 3). These metestrus females also exhibit significantly greater fear recovery during extinction recall (poor extinction memory) compared to males. However, when metestrus females are subcutaneously injected with a 15 μg/kg dose of estradiol prior to extinction learning, consolidation of the fear extinction memory appears to be enhanced as demonstrated by low freezing during extinction recall (Figure 4). Therefore, these data indicate that gonadal hormones can modulate fear extinction recall and also mediate sex differences in conditioned fear extinction behaviors.
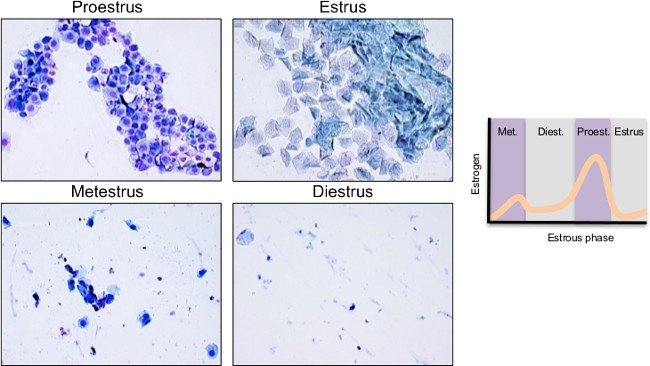
Figure 1: Representative images of cell types across estrous cycle phases (magnification 20X). Proestrus is characterized by masses of round, purple-stained nucleated epithelial cells. Estrus can be identified by aggregates of blue-stained, squamous cornified cells. Metestrus and diestrus express a combination of cell types (leukocytes, nucleated and cornified cells) but show fewer cells. Estrogen levels fluctuate throughout the estrous phases, peaking during proestrus. (Met.= metestrus; Diest. = diestrus; Proest. = proestrus). Please click here to view a larger version of this figure.
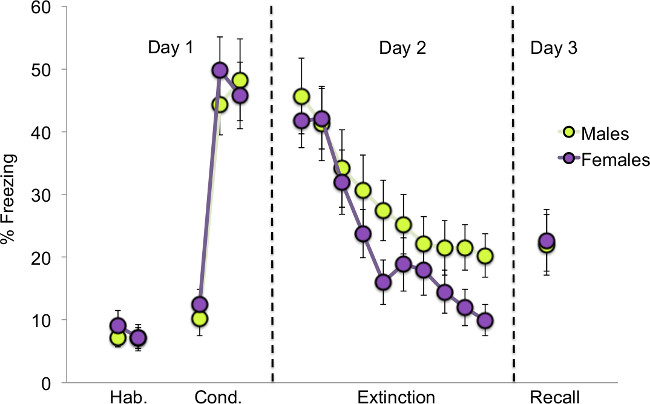
Figure 2: Comparison between males and females across the three phases of the experiment. There are no significant sex differences observed in the habituation (Hab.), conditioning (Cond.), extinction, or recall phases of the experiment15.
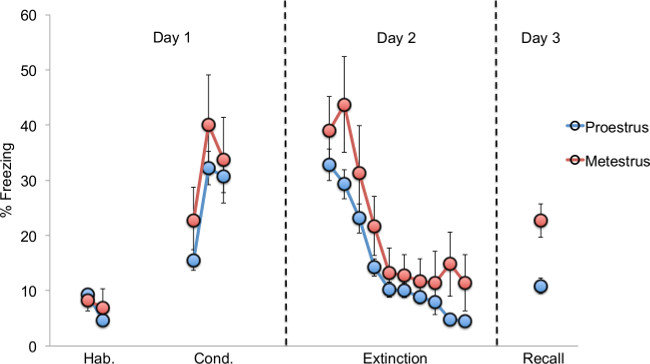
Figure 3: Behavioral differences between proestrus (high estrogen) and metestrus (low estrogen) females. Females in the high-estrogen proestrus phase of the estrous cycle exhibit less fear than those in low-estrogen metestrus. These data suggest that estrogen levels in naturally cycling females can influence the consolidation of fear extinction memory, modulating recall 24 hr later15.
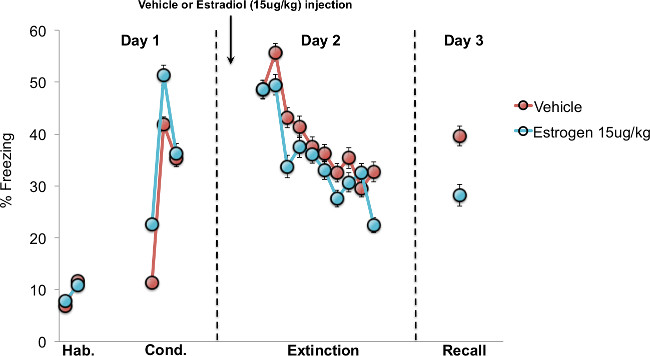
Figure 4: Estrogen treatment in metestrus females. Administration of estradiol 30 min before extinction training (arrow) facilitates extinction recall compared to vehicle16.
Discussion
Fear extinction has been studied extensively in male rats with the neural mechanisms mediating conditioned fear extinction identified and assessed under various manipulations. Relatively few studies have examined female rats or the role of gonadal hormones in fear extinction. To specifically study the effects of estrogen on fear extinction, naturally cycling female rats are subjected to a three-day behavioral paradigm. This procedure consists of habituation/conditioning, extinction, and extinction recall phases. Using this protocol, it has been demonstrated that female rats trained with extinction during the low-estrogen metestrus phase exhibit higher freezing (or impaired extinction recall) compared to females that are extinguished in high-estrogen proestrus15. Administration of estradiol prior to extinction training during the metestrus phase modulates the consolidation of extinction memory16. This is demonstrated by a decrease in freezing during extinction recall. Together, these results highlight the importance of conducting female studies with attention to the influence of ovarian hormones and the estrous cycle on behavior. Although control experiments have not been described here, it will be necessary to include the appropriate controls, i.e. no extinction groups, to further evaluate the influence of estrogen on processes involved in extinction memory consolidation.
The purpose of this protocol is two-fold: 1) to investigate the effects of estrogen on conditioned fear extinction during specific phases of the estrous cycle in intact animals, and 2) to examine how acute estradiol replacement during the female’s normally low estrogen state can modulate its behavior. Through the vaginal swabbing procedure described here, the four phases of the estrous cycle can be identified: estrus, metestrus, diestrus, and proestrus. This allows us to assess behavior when ovarian hormones such as estrogen are naturally at low and high concentrations. For example, estrogen levels are low during the estrus (20-40 pg/ml), metestrus (15-20 pg/ml), and diestrus (25-40 pg/ml) phases, but high during the proestrus (~75 pg/ml) phase of the estrous cycle27,28. These phases not only differ in hormonal milieu but also in duration. Although variable, proestrus has been typically observed to last for 12 to 14 hr; estrus for 25 to 27 hr; metestrus for 6 to 8 hr; and diestrus for 55 to 57 hr. The duration of each phase may be longer or shorter than a day (depending on the animal), leading to 5-day cycles that include 2 days of either diestrus or estrus. As mentioned, proestrus and metestrus are shorter phases (12-14 and 6-8 hr, respectively) and may be missed. In this protocol, it is therefore critical to carefully monitor the estrous cycle when using the animal’s naturally cycling hormones to examine inter-phase differences in the ability to recall extinction training from the day before. The estrous cycle in female rats, much like the human menstrual cycle, is also sensitive to numerous external factors, such as age, stress, time of day, housing conditions, temperature, etc., and should be considered when planning experiments. An animal might become acyclic (extended estrus/diestrus) or display an irregular cycle (shorter than 4 days or longer than 5 days) before or during the study. In this case, these animals would be excluded from the experimental analyses, being strict to testing during specific phases of the estrous cycle. Important to note are transition stages during which the samples are mixed with mucus, making it slightly difficult to clearly identify the specific cell types that characterize each phase. This can also be due to the time of the day the swab is taken. If there is an excessive amount of mucus-like discharge during smear collection as a result of daily swabbing, allowing several handling days to pass without swabbing can resolve this issue.
In addition to the estrous phase identification method described here, other methodological tools can be employed to obtain measures of circulating serum ovarian hormone concentrations. For example, estrogen concentration can be quantified in blood samples using an enzyme-linked immunosorbant assay (ELISA). In this procedure, estrogens within the serum/plasma samples bind to a specific anti-estrogen antibody, which is linked to an enzyme. The enzyme’s substrate produces a reaction that results in a visible signal such as a color change that can be measured using a spectrophotometer. This tool calculates absorbance and provides a quantification of estrogen levels in the sample. This method can be useful in assessing naturally fluctuating estradiol levels across the estrous cycle, and more importantly, at specific times during the experiment. It can also be used to determine how exogenous administration of estrogen can alter normal physiological concentrations. Measurement of estrogen concentrations from blood can prevent difficulties with phase identification during transition stages, or when smears are difficult to read due to mucus in the samples. Unfortunately, this method can become quite costly and offers delayed results compared to vaginal smear collection.
Procedures to obtain sufficient blood samples, e.g., via tail vein, orbital sinus, tail snip, and saphenous vein29, may cause unnecessary stress to the animal. This may impact hormone levels at critical times in the experiment, leading to inaccurate or skewed measurements. Using trunk blood to measure estradiol levels is terminal and therefore provides very limited information about hormonal fluctuations within the phases. Assessing vaginal cell cytology appears to offer a better alternative. Although this method may only provide a rough estimate of ovarian hormone profiles across estrous phases, it can minimize stress and is subsequently less disruptive to the behavioral experiment.
There are different forms of estrogenic steroids that are naturally circulating in humans and non-human animals, such as estriol, estradiol, and estrone. In this protocol, estradiol is acutely administered via subcutaneous injections. Estradiol (synonymous with E2, 17beta-estradiol or beta-estradiol) is used in this protocol, because it is the most potent form of naturally occurring estrogen in non-pregnant females30. In contrast to acute injections of estradiol, other laboratories have employed methods of chronic estrogen administration. For example, subcutaneous implants of slow-release pellets or silastic capsules allow for steady, continuous delivery of estrogen or vehicle solution19. This procedure is typically used in conjunction with ovariectomies, which eliminate the animal’s natural reproductive state.
The manipulations discussed here focus on natural hormonal states to facilitate clinical application of the laboratory findings. Individuals with post-traumatic stress disorder (PTSD) are commonly impaired in their ability to extinguish fear of a stimulus related to their traumatic experience and to remember this extinction. This condition often persists long after the event has passed. Prolonged exposure therapy is a common treatment option for PTSD that relies on extinction-based processes studied in rodent models such as the one described here. Interestingly, women are roughly twice as likely as men to develop stress and anxiety-related psychiatric disorders31. This sex difference in prevalence may be linked to sex differences in fear extinction processes and underlies the potential clinical significance of these laboratory findings. Despite the higher prevalence of PTSD in women, relatively few fear extinction studies in rodents have been conducted in females. Female studies focusing on the role of gonadal hormones should not be overlooked when examining sex differences in behavior. Characterizing the behavioral effects of naturally fluctuating hormone levels across the estrous cycle provides valuable insight into the fundamental differences in behavior between males and females. This protocol provides a basic model to facilitate future studies of ovarian hormones and their influence on, but not limited to, cognitive and behavioral processes such as fear extinction. This protocol focuses on a specific gonadal hormone, estrogen, however, the effects of other hormones such as progesterone and testosterone can also be evaluated. Using the behavioral paradigm presented here in conjunction with other pharmacological, cellular, and molecular techniques (i.e., localized drug infusions, immunohistochemistry, western blot), future research may identify specific sites/targets of estrogen action within the brain that may augment behavioral therapy.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Mohammed R. Milad is supported by NIMH grant R01 MH097880 and institutional funds from the Department of Psychiatry at MGH.
Materials
| Name | Company | Catalog number | Comments |
| Fear conditioning chamber | Coulbourn Instruments | ||
| Graphic State | Coulbourn Instruments | ||
| Sound-attenuating box | Med Associates, Inc. | NIR-022MD | |
| Estradiol | Sigma-Aldrich | E1024 | In sesame oil for subcutaneous injection |
| Sesame oil | Sigma-Aldrich | S3547-250ML | |
| Freezescan | Cleversys, Inc. | ||
| Dip quick stain | Jorgensen Laboratories, Inc. | J0322A1, J0322A2, J0322A3 | |
| Cotton-tipped applicators | Fisher Scientific | 23-400-114 | 6-inch, sterile |
| 0.9% saline | LabChem, Inc. | LC23460-2 | Sodium chloride w/v |
| Selectfrost microscope slides | Fisher Scientific | 12-550-003 | |
| Virex II 256 | Diversey, Inc. | 5019317 | Dilute 1:256 with water |
| Luer-Lok Tip 1ml Syringes | Becton Dickinson | 309628 | |
| Hypodermic disposable needles | Exelint International, Co. | 26402 | 26-gauge |
Riferimenti
- Baron-Cohen, S., Knickmeyer, R. C., Belmonte, M. K. Sex differences in the brain: implications for explaining autism. Science. 310 (5749), 819-823 (2005).
- Voyer, D., Voyer, S., Bryden, M. P. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 117 (2), 250-270 (1995).
- Pinker, S., Spelke, E. A Conversation with Steven Pinker and Elizabeth Spelke. The Science of Gender and Science. , (2005).
- Hampson, E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 15, 97-111 (1990).
- Hampson, E., Kimura, D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behavioral Neuroscience. 102, 456-459 (1988).
- Hampson, E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain and Cognition. 14 (1), 26-43 (1990).
- Kilpatrick, D. G., Resnick, H. S., Milanak, M. E., Miller, M. W., Keyes, K. M., Friedman, M. J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress. 26 (5), 537-547 (2013).
- Breslau, N. Gender differences in trauma and posttraumatic stress disorder. Journal of Gender-Specific Medicine. 5 (1), 34-40 (2002).
- Frans, O., Rimmo, P. A., Aberg, L., Fredrikson, M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatrica Scandinavica. 111 (4), 291-299 (2005).
- Breslau, N., Kessler, R. C., Chilcoat, H. D., Schultz, L. R., Davis, G. C., Andreski, P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 55 (7), 626-632 (1996).
- Seedat, S., Stein, D. J., Carey, P. D. Post-traumatic stress disorder in women: epidemiological and treatment issues. CNS Drugs. 19 (5), 411-427 (2005).
- Holbrook, T. L., Hoyt, D. B., Stein, M. B., Sieber, W. J. Gender differences in long-term posttraumatic stress disorder outcomes after major trauma: women are at higher risk of adverse outcomes than men. Journal of Trauma. 53 (5), 882-888 (2002).
- Labad, J., Menchon, J. M., Alonso, P., Segalas, C., Jimenez, S., Jaurrieta, N., et al. Gender differences in obsessive-compulsive symptom dimensions. Depression and Anxiety. 25 (10), 832-838 (2008).
- Gupta, R. R., Sen, S., Diepenhorst, L. L., Rudick, C. N., Maren, S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Research. 888, 356-365 (2001).
- Milad, M. R., Igoe, S. A., Lebron-Milad, K., Novales, J. E. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscienze. 164 (3), 887-895 (2009).
- Zeidan, M., et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 70 (10), 920-927 (2011).
- Lebron-Milad, K., Milad, M. R. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of Mood & Anxiety Disorders. 2 (3), (2012).
- Rey, C. D., Lipps, J., Shansky, R. M. Dopamine d1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 39 (5), 1282-1289 (2013).
- Ström, J. O., Theodorsson, A., Ingberg, E., Isaksson, I. M., Theodorsson, E. Ovariectomy and 17β-estradiol Replacement in Rats and Mice: A Visual Demonstration. Journal of Visualized Experiments. , e4013 (2012).
- Markham, J. A., Pych, J. C., Juraska, J. M. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Hormones & Behavior. 42 (3), 284-293 (2002).
- Markowska, A. L., Savonenko, A. V. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. Journal of Neuroscience. 22 (24), 10985-10995 (2002).
- Bredemann, T. M., McMahon, L. L. 17β Estradiol Increases Resilience and Improves Hippocampal Synaptic Function in Helpless Ovariectomized Rats. Psychoneuroendocrinology. 42, 77-88 (2014).
- Grueso, R., et al. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxidants and Redox Signaling. 20 (2), 236-246 (2014).
- Shughrue, P. J., Bushnell, C. D., Dorsa, D. M. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 131 (1), 381-388 (1992).
- Frye, C. A., Erskine, M. S. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. Journal of Reproduction and Fertility. 90 (2), 375-385 (1990).
- Becker, J. B., et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 146, 1650-1673 (2005).
- Westwood, F. R. The female rat reproductive cycle: a practical histological guide to staging. Toxicologic Pathology. 36 (3), 375-384 (2008).
- Hurn, P. D., Macrae, I. M. Estrogen as a Neuroprotectant in Stroke. Journal of Cerebral Blood Flow & Metabolism. 20, 631-652 (2000).
- Parasuraman, S., Raveendran, R., Kesavan, R. Blood sample collection in small laboratory animals. Journal of Pharmacology and Pharmacotherapeutics. 1 (2), 87-93 (2010).
- Gillies, G. E., McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological Reviews. 62 (2), 155-198 (2010).
- Tolin, D. F., Foa, E. B. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological Bulletin. 132, 959-992 (2006).

