An Enzymatic Method to Rescue Mesenchymal Stem Cells from Clotted Bone Marrow Samples
Summary
Mesenchymal stem cells are usually obtained from bone marrow and require expansion culture. When samples clot before processing, a protocol using the (enzymatic) thrombolytic drug urokinase can be applied to degrade the clot. Thus, cells are released and available for expansion culture. This protocol provides a rapid and inexpensive alternative to resampling.
Abstract
Mesenchymal stem cells (MSCs) – usually obtained from bone marrow – often require expansion culture. Our protocol uses clinical grade urokinase to degrade clots in the bone marrow and release MSCs for further use. This protocol provides a rapid and inexpensive alternative to bone marrow resampling. Bone marrow is a major source of MSCs, which are interesting for tissue engineering and autologous stem cell therapies. Upon withdrawal bone marrow may clot, as it comprises all of the hematopoietic system. The resulting clots contain also MSCs that are lost for expansion culture or direct stem cell therapy. We experienced that 74% of canine bone marrow samples contained clots and yielded less than half of the stem cell number expected from unclotted samples. Thus, we developed a protocol for enzymatic digestion of those clots to avoid labor-intense and costly bone marrow resampling. Urokinase – a clinically approved and readily available thrombolytic drug – clears away the bone marrow clots almost completely. As a consequence, treated bone marrow aspirates yield similar numbers of MSCs as unclotted samples. Also, after urokinase treatment the cells kept their metabolic activity and the ability to differentiate into chondrogenic, osteogenic and adipogenic lineages. Our protocol salvages clotted blood and bone marrow samples without affecting the quality of the cells. This obsoletes resampling, considerably reduces sampling costs and enables the use of clotted samples for research or therapy.
Introduction
Mesenchymal stem cells (MSCs) play a major role in regenerative medicine and tissue engineering. They can migrate, differentiate into various cell types 1 and engraft, which renders them the ideal candidates for autologous therapies 2,3. Lately, clinical trials using MSCs for bone and cartilage repair, graft versus host disease or heart disease were launched 4. These MSCs can be harvested from the umbilical cord or adipose tissue but most promising results were obtained from bone marrow derived stem cells 5.
The iliac crest allows to collect a considerable amount of bone marrow and therefore serves as main site of aspiration 6. However, the quality of the aspirate decreases with increasing volume of bone marrow withdrawn. While the first 5 ml of bone marrow aspirate contain MSCs of high quality, withdrawal of larger volumes leads to dilution of the aspirate with peripheral blood from the highly vascularized bone 7. Because of the present megakaryocytes and platelets, bone marrow aspirates are prone to clotting, unless anticoagulants are used. But even with anticoagulants, clots may occur.
In bone marrow, MSCs represent only a small proportion of the total cell pool 8 and have to be expanded in culture for most tissue engineering or therapeutic applications 4. The quality of such a culture largely depends on the initial cell pool, i.e., diversity and a high starting number 9. Low numbers of MSCs from withdrawals may be partly explained by donor variability. On the other hand, MSCs from low quality samples require longer time in culture and extended passaging to reach the desired number of cells. In either case, extended passaging is a source of cell senescence and can lead to the loss of differentiation potential 10. Therefore, optimized protocols that can maximize cell yield and prevent from detrimental effects have to be developed 11,12.
When we began to work with canine MSCs, we were astonished to see that about three in four canine bone marrow samples contained clots, while fortunately clotted human samples (one in ten) were less frequent. On the other hand it was no surprise, that we observed much lower yields of MSCs from clotted samples. To solve the recurring issue of clotted samples, we developed the protocol using the thrombolytic drug urokinase instead of resampling.
Thrombolytic therapies can counteract life threatening situations such as occlusion of blood vessels causing heart attack, stroke or embolisms because of unwanted clotting. They work by degradation of the clots through enzymatic cleavage of fibrin by plasmin and enzymatic plasminogen activators. Despite the wide use for treatment of patients, only very few publications exist that utilized thrombolytic activities for laboratory applications to rescue clotted samples, mostly focusing on lymphocytes. In 1987, Niku et al. described the use of streptokinase for dissolving blood clots resulting in functional lymphocytes 13 and four years later, De Vis et al. extended the use of streptokinase to isolate leukemia cells from blood and bone marrow for flow cytometric applications 14. A more recent publication suggests the use of Alteplase for cancer diagnostics 15. While using the same enzymatic approach, our protocol focuses on the isolation of multipotent MSCs form bone marrow to provide a tool for researchers in the stem cell field.
Protocol
NOTE: Human bone marrow aspirates from the iliac crest were collected from consenting donors with the approval of the ethics committee of the canton of Lucerne. Canine bone marrow aspirates from the iliac crest were collected with dog owner’s consent.Human (approx. 20 ml) and canine (approx. 10 ml) bone marrow aspirates were anti-coagulated by addition of 15 ml of 3.8% sodium citrate immediately after withdrawal in the operation theatre. The samples were transferred to the laboratory environment for processing the same day as withdrawn.
1. Preparation of Urokinase (Prior to 1st Use)
- Reconstitute urokinase using sterile phosphate buffered saline (PBS) according to the manufacturer’s instructions: Add 10 ml PBS to the septum-inlet flask using a 10 ml syringe. Dissolve the dry substance by swirling to obtain 50,000 injection units (U) per ml.
- Prepare 500 µl aliquots (25,000 U each) into sterile tubes. Store aliquots -20 °C and use it when required for at least 6 months.
2. Preparative Steps (Prior to Every Bone Marrow Treatment)
- Thaw one aliquot of urokinase (25,000 U) per clotted bone marrow aspirate (aspirate volumes up to 25 ml have been successfully treated using a single aliquot of 25,000 U).
- Preheat a water bath or shaking incubator to 37 °C.
3. Enzymatic Digest of the Clot
- Perform all work in a laminar flow biosafety cabinet to avoid microbial contamination of the bone marrow samples during processing. When working with potentially infectious human material, the use of protective gloves as well as careful handling is advised.
- Place a 100 µm cell strainer on top of a sterile 50 ml tube. Carefully pour the bone marrow aspirate through the cell strainer, tilting the strainer or moving around the clot material. Use a sterile pipet tip for better flow through the filter mesh.
NOTE: Avoid any dilution of the clot, e.g., by washing the clot with PBS. Urokinase acts indirectly and requires components of the serum (i.e., plasminogen) from the biopsy for effective digest. While continuing the procedure with the clot material, the filtrate can be kept at RT until further use in step 3.6. - Transfer the bone marrow clot material into an empty cell culture dish using sterile forceps. Cut the debris into small pieces of approximately 2 mm3 using a sterile scalpel.
- Transfer the small pieces of the clot into a 50 ml reaction tube using the sterile forceps. Ensure that the minced clot has a wet appearance. If it appears to be dry, use some of the filtrate from step 3.1 to moisten.
- Triturate the clot by pipetting up and down for 5 times using a 5 ml microtiter pipet. Add 1 aliquot of urokinase to the sample. Incubate for 30 min at 37 °C either in a water bath or in a shaking (gently) incubator.
- Triturate by pipetting up and down for 5 times using a 5 ml pipet. Perform this step in a biosafety cabinet. Incubate for another 30 min at 37 °C. After the incubation, triturate again 5 times using a 5 ml pipet. Pass over a fresh 100 µm cell strainer to pool with the filtrate from step 3.2.
- Centrifuge the cell suspension at ambient temperature for 10 min at 500 x g. Discard the supernatant. Re-suspend the cell pellet in media as described below or according to the standard procedure of your laboratory for MSC expansion culture.
4. Seeding for Expansion Cell Culture and CFU Plates
- Resuspend the cells (consisting of erythrocytes and mononuclear cells) in 50 ml of basal medium: Alpha-MEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin and 2.5 mg/ml amphotericin B. Count the cells diluted 1:10 in Trypan Blue solution using a Neubauer chamber.
- Plate the cells in cell culture flasks at the density of 5 x 107 cells/cm2 and incubate in a humidified incubator at 37 °C. If available, use hypoxic (5% oxygen) conditions. For the CFU assay control, plate 109 cells in a cell culture dish of 10 cm diameter.
- After 3 days of culture, mesenchymal stem cells are attached to the cell culture dish while other cells remain in suspension. Change the media with basal medium supplemented with 5 ng/ml basic fibroblast growth factor (bFGF). For the CFU assay control, treat the cell culture dish likewise.
- Continue cell culture for a total of 2 weeks, exchanging media three times a week. If cells exceed 80% confluency, split by trypsinization. For the CFU assay control, exchange the media likewise, but do not split the cells for the course of the two weeks.
5. Giemsa Stain for CFU Assay
- Prepare 10 ml of Giemsa-solution per dish: dilute the stock solution (7.6 g/l Giemsa in glycerol:methanol) 1:10 in sterile water (always prepare a fresh working dilution).
- Remove the media from the cell culture dish and wash the cells with PBS. Be very precautious when applying liquid to the petri dish and avoid washing off the cells.
- Fix cells in pure Methanol for 5 min at RT. Discard the Methanol. Add the Giemsa-solution and incubate for 60 min in a humidified incubator at 37 °C.
- Wash two times with PBS. Air dry the plate head first on a paper towel. Count the colonies manually. This is best achieved using a marker pen on the back of the plate.
6. MSC Differentiation
- Canine MSCs differentiation
NOTE: Canine MSCs were differentiated into chondrogenic, osteogenic and adipogenic lineages by stimulation with the appropriate media for four weeks. - For Adipogenic differentiation, culture MSCs in monolayer at 4 x 105 cells/cm2 alternating two different culture conditions as follows;
- Culture in adipogenesis maintenance media containing DMEM + GlutaMAX, 3% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, 2.5 µg/ml amphotericin B and 170 mM insulin.
- Culture in adipogenesis inducing media with maintenance medium supplemented with 3% FBS, 5% rabbit serum, 1 µM dexamethasone, 500 µM 3-Isobutyl-1-methylxanthine, 33 µM biotin, 5 µM rosiglitazone and 17 µM pantothenate 16.
- Revel the lipid droplets by staining with Oil Red-O. Briefly, fix cells with 10% formaldehyde, wash with PBS and stain with 0.35% Oil red O in isopropanol.
- For Osteogenic differentiation culture MSCs in monolayer at 7 x 103 cells/cm2 and stimulate in the following:
- Advanced DMEM (GIBCO) + GlutaMAX, 5 % FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, 2.5 µg/ml amphotericin B, 50 µM L-ascorbic acid 2-phosphate, 10 mM ß-glycerophosphate and 100 nM dexamethasone.
- Identify the mineralization deposits by Von Kossa stain (5 % AgNO3). Briefly, fix the cells with 10% formaldehyde, wash with PBS and stain with 5 % AgNO3in distilled water.
- Chondrogenic differentiation
- Cut cubes (3 mm per side) from a sponge shaped medical device, constituted from lyophilized collagen type I, and used as scaffold material to support cells 17.
- Seed MSCs on the top of the cubes at the concentration of 4 x 106 cells/ml (~70,000 cells/cube).
- Prior to the addition of media, allow the cells to adhere to the cubes for 30 min.
- Maintain MSC-collagen constructs in chondrogenic media consisting of DMEM/F12 + GlutaMAX, 2.5 % FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, 2.5 µg/ml amphotericin B, 40 ng/ml dexamethasone, 50 µg/ml ascorbic acid 2-phosphate, 50 µg/ml L-proline, 1x Insulin-Transferrin-Selenium X, and 10 ng/ml transforming growth factor-β1.
- Use alcian blue staining to visualize accumulation of proteoglycans in constructs sections. Briefly, stain the sections O/N with 0.4% alcian blue dissolved in 0.01% H2SO4 and 0.5M guanidine hydrochloride. Next, wash the sections were washed for 30 min in 40% DMSO and 0.05M MgCl2. Cells were counterstained with nuclear fast red.
Representative Results
The facts that 74% of canine bone marrow samples (n=54) contained clots when they arrived in our laboratory (Figure 1A) along with decreased MSC yields from these samples, made us believe that a considerable number of MSCs was trapped within the clots. Indeed, a simple DAPI-stain of sectioned clot material confirms the presence of nucleated cells in high density (Figure 1B). This ultimately leads to low numbers of MSCs available for expansion culture, which triggered us to develop the protocol using urokinase. Typically clots disappear almost completely when applying our protocol. However, during method development we addressed the question of clot digest systematically by weighing before and after digest. These experiments revealed that the undigested remainders were 15% (dog) or 9% (human) of the initial clot weight (Figure 2A).
A typical feature of bone marrow derived MSCs is the ability to adhere to cell culture dishes. Researchers make use of this characteristic to select for MSCs. As a consequence, a colony forming assay allows to evaluate the quality of the cell pool in a simple, quantitative and reliable way. In our laboratory, the colony forming assay described herein has been applied routinely to all bone marrow samples processed (also non-clotted ones). This allowed us to use the assay as main criterion for determining the efficacy of the urokinase digest. To allow direct comparison, cells from clotted sample filtrates (i.e. as if clotted samples were just filtered but not enzymatically treated) and digested clots were seeded on separate 10 cm dishes followed by incubation for two weeks. When visualizing colony forming units (CFU) with Giemsa stain (as shown in Figure 2B), we observed 3.8 times more CFU from the urokinase reactions of canine samples than from the corresponding initial filtrates (Figure 2C). Although less prominent, human samples followed a similar trend with 1.6 fold more CFU from treated samples, confirming the suitability of the protocol. Indeed, the plates of digested clot illustrated in the right row in Figure 2B correspond to the typical result seen for a pooled sample. However, donor variation may easily result in CFU numbers from half to double of what is depicted.
Taking the colony-size as indicator of cell pool quality, more medium (2-5 mm) and large (>5 mm) colonies appeared on the plates seeded from clots compared to the CFU from filtrates (Figure 2C). This generally indicates that MSCs grow normally after urokinase treatment. Also in the aggregate, the comparison of digested canine specimens (n = 21) to samples without clot (n = 7) yielded comparable numbers of total MSCs for treated samples, although a big inter-sample variation was observed due to the heterogeneous donor population (Figure 2D).
As a last functional test of suitability, we induced differentiation of MSCs derived from digested bone marrow samples. Applications in autologous stem cell therapies are based on MSC differentiation into cartilage, bone or adipose tissue or MSC paracrine signaling 18. Cells can be guided towards the desired path of differentiation by choosing the appropriate culture conditions for adipogenic differentiation 19, osteogenic differentiation 20 or chondrogenic lineage 17. Hence, we compared MSCs from bone marrow clot to MSCs derived from unclotted bone marrow sample. After four weeks in culture, it was possible to differentiate MSCs into all three above mentioned lineages. This was histologically tested with Von Kossa (for osteogenic lineage), Oil Red O (adipogenic) and Alcian Blue (chondrogenic) stainings, showing no differences in the grade of differentiation between the groups (Figure 3).
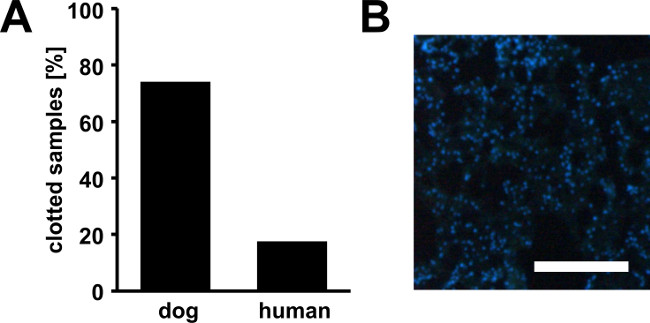
Figure 1. Bone marrow clots. Percentage of canine and human bone marrow samples in partially clotted state upon arrival (A). Furthermore, we found that MSC yields from clotted samples were strongly reduced due to a high number of cells trapped within the clots. A high number of nucleated cells are present within the clots as demonstrated by DAPI-staining of a cryosection from a canine bone marrow clot (B). Scale bar represents 200 µm.
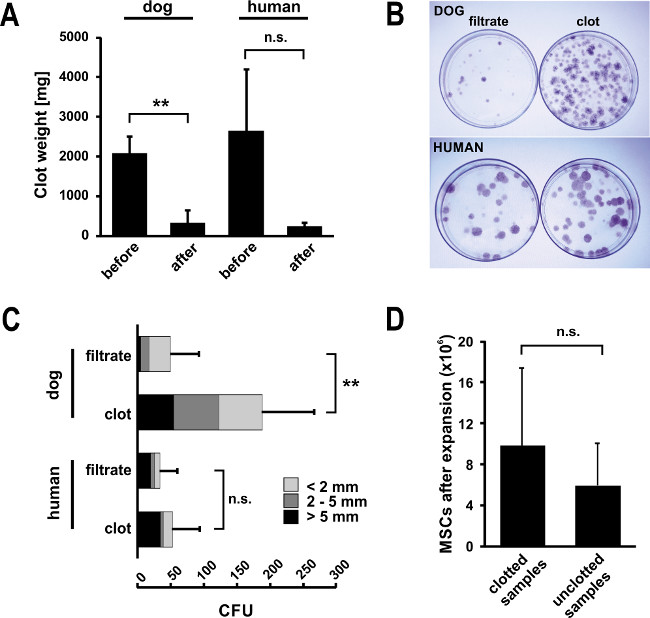
Figure 2. Isolation of MSCs from bone marrow clots digested with urokinase. (A) Typically, bone marrow clots disappear almost completely upon urokinase digest. Upon method development, we assessed clot weights for canine (n = 5, mean ± SD, **p ≤0.01) and human samples (n = 3, mean ± SD, n.s. = not significant p = 0.10) that were strongly reduced upon urokinase digest. (B) A simple CFU assay can serve as an informative tool for assessing the quality of an MSC preparation. To assess the efficacy of the digest, the CFU assay was performed for filtrates and digested clots from bone marrow aspirates separately. After two weeks in culture, 10 cm control plates were stained with GIEMSA and CFU were counted. The assay confirmed that high number of CFU can be released from the clot by urokinase digest and remain functional. This is also confirmed by the high frequency of large colonies (class-division: >5 mm in black, 2-5 mm in dark grey and <2 mm in light grey. Error bars represent SD of total CFU (n = 5 for dog, n = 3 for human, **p ≤0.01, n.s. = not significant, p = 0.17). (C) Pictures from Giemsa-stained plates confirm the results shown. The plates shown for digested clot correspond to a typical result for a digested bone marrow sample – however, numbers can vary largely due to donor variability. (D) In the sum, applying the urokinase digest protocol (n = 21) results in comparable total MSC yields after expansion culture to naturally clot free samples (n = 7, mean ± SD). Statistical analysis for the entire Figure 2 was performed using Student’s t-test.
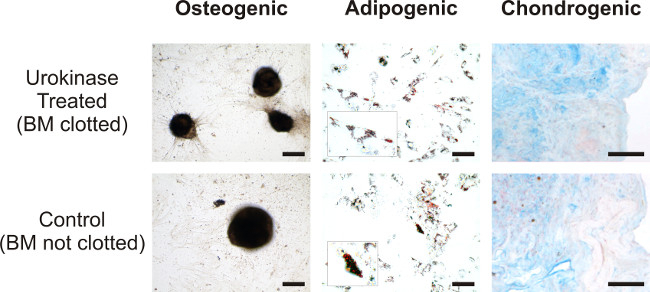
Figure 3. Comparison of differentiation potential of digested versus natively unclotted samples. Canine MSCs isolated from clotted bone marrow treated with Urokinase (top row) and unclotted bone marrow (bottom row) were differentiated in osteogenic phenotype and stained by Von Kossa (bar = 200 µm), adipogenic phenotype was stained by Oil Red O (bar =100 µm; in the insets larger magnification of fat vacuoles), while chondrogenesis was revealed by Alcian blue staining (bar =100 µm; counterstaining nuclear fast red). Please click here to view a larger version of this figure.
Discussion
Routinely we sample bone marrow while the patient is undergoing surgery (in our case mainly spine surgery), with the advantage that only little additional work has to be carried out by the personnel in the operation theatre. Even though the samples are mixed with sodium citrate immediately after withdrawal, many samples were partially clotted when they arrived in the laboratory for processing. At this stage, resampling to replace clotted specimens would be a separate additional intervention necessitating again local or general anesthesia 6. This requires the willingness of both the clinical staff and the donor to contribute and consumes a lot of resources 21.
Here we provide a protocol that uses recombinant human urokinase on clotted bone marrow samples to isolate multipotent MSCs. We could demonstrate that the urokinase treatment does not harm the cells and the differentiation potential is retained. The protocol is short since it prolongs sample processing only by approximately 1 ½ hr as compared to unclotted samples while yielding comparable cell pools. To date, this protocol has been successfully applied on bone marrow clots from different individuals and dogs using urokinase from several lots and has shown robust and reproducible success. Apart from this, urokinase acts indirectly via activation of sample-intrinsic plasminogen. This implies that the urokinase reaction requires the plasma-environment. It is therefore unadvisable to introduce steps of clot rinsing or alike with e.g., PBS. This would dilute the serum environment and lead to a significant slow-down of the enzymatic reaction.
The decision to use urokinase for our method and not another thrombolytic drug was trivial: we developed the protocol based on the thrombolytic drug readily available in our hospital pharmacy. For other research groups it may therefore be easier to use a tissue-type plasminogen activator (i.e., a different drug product like alteplase, reteplase or tenecteplase) if urokinase is not available. However, potentially important differences between the drugs exist: unlike tissue-type plasminogen activators, urokinase can trigger cell activation and proliferation when bound to the urokinase-type plasminogen activator receptor (uPAR) 22. Upon binding, intracellular signaling cascades are activated that lead to cell migration and proliferation. In a physiological situation this is further supported by the secretion of matrix metalloproteinases by activated cells. However, in our system, urokinase is diluted and gradually removed upon expansion culture without showing detrimental effects. Still, with respect to the intended use of isolated MSCs, the suitability of any of the thrombolytic drugs needs to be determined individually.
Generally, high dosages for rapid and complete clot digest are recommended to minimize unwanted selection during clot processing. Noteworthy, many previous studies used bacterial streptokinase for digesting blood and bone marrow clots 13,14. However, this drug may provoke unwanted activation and response of the immune system, which may be a major drawback especially when application of cells to patients is intended.
We therefore believe that our protocol does not only help researchers and medical professionals to save time and reduce costs. Using urokinase – an approved enzymatic drug without known antigenicity – may even provide a valuable tool for many translational research laboratories aiming at therapeutic use of MSC preparations.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Swiss National Foundation Grant CR3I3_140717/1 and the Swiss Paraplegic Foundation.
Materials
| Basal Medium Components | ||
| PenStrep 100X | Gibco | 15140122 |
| Human FGF-basic | Peprotech | 100-18B |
| MEM Alpha w/ Nucleoside, w/ stable Glutamine | Amimed | 1-23S50-I |
| FBS Heat Inactivated | Amimed | 2-01F36-I |
| Amphotericin B | Applichem | A1907 |
| Adipogenic Medium Components | ||
| DMEM-HAM F12 + GlutaMAX | Amimed | 1-26F09-I |
| Insulin | Sigma | I5500 |
| Rabbit serum | Gibco | 16120099 |
| Dexamethasone | Applichem | D4902 |
| 3-Isobutyl-1-methylxanthine | Sigma | I5879 |
| Biotin | Sigma | B4639 |
| Rosiglitazone | Sigma | R2408 |
| Pantothenate | Sigma | P5155 |
| Oil Red-O | Sigma | O0625 |
| Osteogenic Medium Components | ||
| L-ascorbic acid 2-phosphate | Sigma | A8960 |
| ß-glycerophosphate | Sigma | G9422 |
| Silver nitrate (AgNO3) | Sigma | S6506 |
| Chondrogenic Medium Components | ||
| Biopad – sponge shaped medical device | Euroresearch | |
| L-proline | Sigma | P5607 |
| Insulin-Transferrin-Selenium X | Gibco | 51500056 |
| Human transforming growth factor-β1 | Peprotech | 100-21 |
| Alcian Blue 8GX | Sigma | A3157 |
| Nuclear fast red | Sigma | N8002 |
| Generic | ||
| Tri-Sodium citrate dihydrate | Applichem | A3901 |
| PBS | Applichem | 964.9100 |
| Urokinase | Medac | 1976826 |
| 0.5% Trypsin-EDTA | Gibco | 15400054 |
| Giemsa stain | Applichem | A0885 |
| Formaldehyde | Applichem | A0877 |
| Sulfuric acid (H2SO4) | Applichem | A0655 |
| Dimethyl sulfoxide (DMSO) | Applichem | A1584 |
| Magnesium chloride (MgCl2) | Applichem | A3618 |
| Guanidine hydrochloride | Applichem | A1499 |
| Consumables | ||
| 50 mL reaction tube | Axygen | SCT-50ML-25-S |
| 10 mL syringe | Braun | 4606108V |
| Sterican needle (22G) | Braun | 4657624 |
| 1.7 mL Microtubes | Brunschwig | MCT-175-C |
| 100 μm cell strainer | Falcon | 6.05935 |
| sterile forceps | Bastos Viegas, SA | 489-001 |
| sterile scalpel | Braun | 5518059 |
| Primaria cell cuture dish | Falcon | 353803 |
| C-Chip Neubauer Improved | Bioswisstech | 505050 |
| cell culture flask – Flask T300 | TPP | 90301 |
| Equipment | ||
| Microbiological biosafety cabinet class II | Skan | 82011500 |
| water bath | Memmert | 1305.0377 |
| Stripettes Serological Pipette 5ml | Corning | 4487-200ea |
| microscope | Olympus | CKX41 |
| humidified incubator Heracells 240 | Thermo scientific | 51026331 |
| Heraeus Multifuge 1S-R | Thermo scientific | 75004331 |
Riferimenti
- Jiang, Y., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 418 (6893), 41-49 (2002).
- Uccelli, A., Moretta, L., Pistoia, V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 8 (9), 726-736 (2008).
- Bartholomew, A., et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 30 (1), 42-48 (2002).
- Wang, S., Qu, X., Zhao, R. C. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 5, 19 (2012).
- Baksh, D., Song, L., Tuan, R. S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 8 (3), 301-316 (2004).
- Malempati, S., Joshi, S., Lai, S., Braner, D. A., Tegtmeyer, K. Videos in clinical medicine. Bone marrow aspiration and biopsy. N Engl J Med. 361 (15), e28 (2009).
- Cuthbert, R., et al. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy. 14 (4), 431-440 (2012).
- Veyrat-Masson, R., et al. Mesenchymal content of fresh bone marrow: a proposed quality control method for cell therapy. Br J Haematol. 139 (2), 312-320 (2007).
- Lazarus, H. M., Haynesworth, S. E., Gerson, S. L., Rosenthal, N. S., Caplan, A. I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 16 (4), 557-564 (1995).
- Bertolo, A., et al. An in vitro expansion score for tissue-engineering applications with human bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med. , (2013).
- Casiraghi, F., Remuzzi, G., Abbate, M., Perico, N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev. 9 (1), 65-79 (2013).
- Bonab, M. M., et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 7, 14 (2006).
- Niku, S. D., Hoon, D. S., Cochran, A. J., Morton, D. L. Isolation of lymphocytes from clotted blood. J Immunol Methods. 105 (1), 9-14 (1987).
- De Vis, J., Renmans, W., Segers, E., Jochmans, K., De Waele, M. Flow cytometric immunophenotyping of leukemia cells in clotted blood and bone marrow. J Immunol Methods. 137 (2), 193-197 (1991).
- St Antoine, A., et al. Application of thrombolytic drugs on clotted blood and bone marrow specimens to generate usable cells for cytogenetic analyses. Arch Pathol Lab Med. 135 (7), 915-919 (2011).
- Kisiel, A. H., et al. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 73 (8), 1305-1317 (2012).
- Bertolo, A., et al. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur Spine J. 21, S826-S838 (2012).
- Makridakis, M., Roubelakis, M. G., Vlahou, A. Stem cells: insights into the secretome. Biochim Biophys Acta. 1834 (11), 2380-2384 (2013).
- Benvenuti, S., et al. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Invest. 30 (9), RC26-RC30 (2007).
- Jaiswal, N., Haynesworth, S. E., Caplan, A. I., Bruder, S. P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 64 (2), 295-312 (1997).
- Patel, A. Tissue banking for research–bench to bedside and back–myth, reality or fast fading reality at the dawn of a personalised healthcare era. Cell Tissue Bank. 12 (1), 19-21 (2011).
- Smith, H. W., Marshall, C. J. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 11 (1), 23-36 (2010).

