Facile and Efficient Preparation of Tri-component Fluorescent Glycopolymers via RAFT-controlled Polymerization
Summary
An efficient, three-step synthesis of RAFT-based fluorescent glycopolymers, consisting of glycomonomer preparation, copolymerization, and post-modification, is demonstrated. This protocol can be used to prepare RAFT-based statistical glycopolymers with desired structures.
Abstract
Synthetic glycopolymers are instrumental and versatile tools used in various biochemical and biomedical research fields. An example of a facile and efficient synthesis of well-controlled fluorescent statistical glycopolymers using reversible addition-fragmentation chain-transfer (RAFT)-based polymerization is demonstrated. The synthesis starts with the preparation of β-galactose-containing glycomonomer 2-lactobionamidoethyl methacrylamide obtained by reaction of lactobionolactone and N-(2-aminoethyl) methacrylamide (AEMA). 2-Gluconamidoethyl methacrylamide (GAEMA) is used as a structural analog lacking a terminal β-galactoside. The following RAFT-mediated copolymerization reaction involves three different monomers: N-(2-hydroxyethyl) acrylamide as spacer, AEMA as target for further fluorescence labeling, and the glycomonomers. Tolerant of aqueous systems, the RAFT agent used in the reaction is (4-cyanopentanoic acid)-4-dithiobenzoate. Low dispersities (≤1.32), predictable copolymer compositions, and high reproducibility of the polymerizations were observed among the products. Fluorescent polymers are obtained by modifying the glycopolymers with carboxyfluorescein succinimidyl ester targeting the primary amine functional groups on AEMA. Lectin-binding specificities of the resulting glycopolymers are verified by testing with corresponding agarose beads coated with specific glycoepitope recognizing lectins. Because of the ease of the synthesis, the tight control of the product compositions and the good reproducibility of the reaction, this protocol can be translated towards preparation of other RAFT-based glycopolymers with specific structures and compositions, as desired.
Introduction
In the past two decades, investigations with synthetic glycopolymers have undergone slow but continual development, demonstrating significant potential in examining infectious mechanisms that include research which focuses on lectin recognition processes1-3. Since synthetic glycopolymers possessing multivalent sugar moieties exhibit much higher lectin-binding efficacies, as compared to monovalent carbohydrates, they are of great demand in the glycobiology field3. Of particular interest in clinical research is the use of fluorescent glycopolymers to characterize the lectin-mediated bacterial binding with carbohydrates available on human respiratory cell surfaces and mucous glycoprotein. Early in vitro studies employed commercially available polyacrylamide-based glycopolymers in bacterial binding tests. Several of these probes showed promising results, but raised concerns regarding, obtainability, and lot-to-lot variances in both polymer molecular weight and glycoepitope content. An economical in-laboratory protocol was developed which would provide for a satisfactory control of structure content, size, and purity of synthetic glycopolymers targeting bacterial lectins.
In search for a suitable synthetic approach to glycopolymers, a relatively new polymerization technique was tested using a type of controlled radical polymerization that employed reversible addition-fragmentation chain-transfer (RAFT) agents4. Such RAFT reagents have recently been used in a few glycopolymer preparations5-7. Compared with other glycopolymer preparation protocols, RAFT-mediated polymerizations demonstrate several advantages, including the tolerance to a variety of monomer structures and reaction conditions, potential compatibility with aqueous solutions, and low size dispersity of the desired polymeric products8,9. Of notable interest are protocols for preparation of RAFT-based tri-component glycopolymers, allowing control of compositions of different monomers, each of which may have distinct functions10-13. However, most of the previous research endeavors either lacked anomeric pendant carbohydrates10, or employed stepped polymerizations resulting in tri-block copolymers, that consist of covalently linked homopolymers, which often serve different purposes than statistical polymers which are copolymers in which the sequence of monomer residues follow a statistical rule9-13.
Recently, employing the thiocarbonylthio RAFT compound (4-cyanopentanoic acid)-4-dithiobenzoate in an aqueous environment, the preparation of a group of RAFT-based linear tri-component statistical glycopolymers containing specific pendant sugars and their application in lectin-mediated bacterial binding tests was reported14. The overall goal of this method, presented in a visual manner, is to prepare tri-component statistical fluorescent glycopolymers via RAFT-controlled copolymerization. Because of the ease of the one-step polymerization protocol, the fine control over the polymer length and compositions, and the high reproducibility of the reaction, this protocol can be readily applied to other RAFT-based syntheses of glycopolymers with desired structures.
Protocol
1. Synthesis of Glycomonomer 2-Lactobionamidoethyl Methacrylamide
- Dissolve 2 g of lactobionic acid in 3.0 ml of anhydrous methanol and slowly add absolute ethanol in a drop wise fashion until the solution just turns cloudy, then remove the solvents via rotoevaporation.
- Dissolve the residue, from step 1.1, in 3.0 ml anhydrous methanol and, once again, slowly add absolute ethanol until just cloudy, then evaporate the solvents via rotoevaporation. Repeat this step 3 times to obtain lactobiono-1,5-lactone (1.94 g, 98% yield). This product is of sufficient purity to be used in the following reactions.
- Add 1.0 g of lactobionolactone in 3.0 ml of methanol to N-(2-aminoethyl) methacrylamide (AEMA, 0.58 g) and hydroquinone monomethyl ether (MEHQ, 1.0 mg), an inhibitor of self-polymerization, in 2.0 ml of methanol, followed by 1.0 ml of triethylamine. Stir at RT for 48 hr.
- Add 20 ml of deionized H2O (dH2O) to the reaction flask, then remove the methanol and free triethylamine by evaporation to dryness via rotoevaporation.
- To remove any remaining lactobionic acid, add 20 ml of dH2O, then pass the aqueous solution through an anion exchange column (OH– form, 10 mm x 20 mm) into a receiving beaker containing 1.0 mg of MEHQ.
- Remove triethylamine, produced in Step 1.5, by evaporating to dryness via rotoevaporation.
- Add 20 ml of dH2O and remove unreacted AEMA by slowly adding 1 mg aliquots of cation exchange resin (H+ form) until no ninhydrin reactive materials are detectable. Monitor the removal by taking 1 µl aliquots of the solution after each resin addition, applying it to a thin-layer chromatography plate, then spraying the plate with a 2% ninhydrin in ethanol solution. When no deep blue color is observed to develop when the plate is heated to 90 °C for 1 min, the end point has been reached.
- Filter the solution through a fritted glass funnel, transfer the filtrate to a test tube, freeze the sample at -80 °C, and then lyophilize.
- Remove MEHQ from the sample by dissolving the freeze-dried material in a minimum amount of methanol (~0.5 ml), then add cold anhydrous acetone (-20 °C, 15 ml) to precipitate the product. Collect the precipitate by filtration using a fritted glass funnel, then dry the precipitate in a desiccator under vacuum to obtain 2-lactobionamidoethyl methacrylamide (LAEMA) as an off-white powder (0.94 g, 68% yield). This product is of sufficient purity to be used in the following reactions.
2. Synthesis of Monomer 2-Gluconamidoethyl Methacrylamide
Note: The preparation of 2-gluconamidoethyl methacrylamide (GAEMA), which does not possess a pendant sugar, was adapted from a published method15.
- Add 2.0 g of AEMA dissolved in 10 ml of methanol to a solution of D-gluconolactone (1.6 g) in 30 ml of methanol and, with stirring, slowly add 1.6 ml of triethylamine.
- Stir the reaction at RT for 24 hr.
- Filter the precipitated product using a fritted glass funnel and rinse the precipitate three times with 10 ml each of isopropanol, then wash with 10 ml of dry acetone. Dry the precipitated product in a desiccator under vacuum.
3. RAFT Glycopolymer Synthesis
- To remove the inhibitor MEHQ present in commercial N-(2-hydroxyethyl) acrylamide (HEAA), add 1 ml of HEAA to a 2 ml microcentrifuge tube, followed by adding 0.5 g of aluminum oxide nanoparticles. Centrifuge the tube at 300 x g for 30 sec, and use the top layer HEAA in the following reaction.
- Carefully add 32.8 mg of LAEMA (70.0 µmol), 1.7 mg of AEMA (10.5 µmol) and 27.5 µl of HEAA (270 µmol), all dissolved in 0.4 ml of dH2O, to a well-cleaned 1 ml Schlenk tube, thus having a monomer molar ratio of 20:3:77.
- In a parallel reaction, in order to produce control polymers that do not possess any pendant sugar, in lieu of using LAEMA in step 3.2, substitute 21.4 mg of GAEMA (70.0 µmol) in the reaction.
- To the respective Schlenk tube (i.e., 3.2 or 3.3), sequentially add 50 µl of DMF containing 0.53 mg of (4-cyanopentanoic acid)-4-dithiobenzoate (1.9 µmol, RAFT agent), and 50 µl of DMF containing 250 µg of 4,4′-azobis-(4-cyanovaleric acid) (0.9 µmol, initiator). Gently mix by finger tapping.
- Freeze the contents contained in the Schlenk tube employing a dry ice:ethanol bath (75 g dry ice in 100 ml ethanol), apply a vacuum to within 10-50 mTorr, then close the Schlenk valve and permit the solution to slowly thaw to RT. Repeat this freeze–evacuate–thaw cycle twice more. Ensure that all reagents are dissolved after the last thaw.
- Place the Schlenk tube into a sealable plastic bag, evacuate the bag’s air, and then seal it. Transfer the bag containing the Schlenk tube to a water bath preheated at 70 °C and incubate for 24 hr.
- Carefully transfer the solution in the Schlenk tube to a prepared dialysis bag (MWCO = 3,500), and dialyze against dH2O (10 x 2 L) for 24 hr, changing the dH2O every hour for the first 8 hr. Following dialysis, transfer the sample from the dialysis tubing to a test tube, freeze the sample at -80 °C, and then lyophilize it.
Note: The resultant statistical poly-methacrylamide/acrylamide (PMA) copolymers containing pendant 4-O-β-D-galactopyranosyl-D-gluconamide (lactobionamide) (from Step 3.2) or D-gluconamide (from Step 3.3), respectively, are obtained. For convenience of discussion, these two glycopolymers are abbreviated as PMA-LAEMA and PMA-GAEMA, respectively.
4. Post-modification of Glycopolymers with Fluorophores
- Dissolve 5.0 mg of glycopolymer PMA-LAEMA or PMA-GAEMA containing ~0.9 µmole of primary amine functional groups in 0.9 ml of phosphate buffered saline (PBS, 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5), respectively.
- Slowly add 0.6 mg of carboxyfluorescein succinimidyl ester in 100 µl of DMF to the solutions with rapid stirring. Gently stir the reactions for 16 hr in the dark at RT.
- While protected from light, load the sample into a prepared dialysis tubing (MWCO = 3,500) and dialyze against dH2O (2 L) for 16 hr, changing the dialysis solution every hour for the first 8 hr. Following dialysis, transfer the sample from the dialysis tubing to a test tube, freeze the sample at -80 °C, and then lyophilize it.
Note: Following lyophilization, fluorescent glycopolymers PMA-LAEMA-Fluorescein and PMA-GAEMA-Fluorescein, respectively, are obtained.
5. Characterization of the Glycopolymers
- Determine the number average molecular weight (Mn), the weight average molecular weight (Mw) and the dispersity (Mw/Mn) of the glycopolymers on a commercial HPLC system equipped with gel permeation chromatography (GPC) software, a GPC column suitable for the molecular weight of interest, and a refractive index detector, using 0.1 M Tris/0.1 M sodium chloride buffer (pH 7) as eluent at a flow rate of 0.6 ml/min14. Use polyethylene glycol standards as molecular weight standards (MW: 200-1,200,000 g/mol).
- Quantify the actual concentrations of primary amine functional groups within the glycopolymers16. Analyze the total carbohydrate content of the synthesized glycopolymers according to a published method17.
- Perform tests of structural composition and purity of the glycomonomers LAEMA, GAEMA and glycopolymers PMA-LAEMA, PMA-GAEMA in D2O by NMR spectroscopy14.
6. Binding Tests of the Synthetic Glycopolymers with Lectin-coated Agarose Beads
- Add 1.5 ml of PBS to 50 µl of suspension of Erythrina crista-galli lectin (ECL)-coated agarose beads, centrifuge at 300 x g for 1 min, and carefully remove and dispose of the supernatant. Repeat this step twice, and then resuspend the beads in 0.5 ml of PBS.
- Add 3 µg of PMA-LAEMA-Fluorescein or PMA-GAEMA-Fluorescein (negative control) in 6 µl of PBS to the beads suspension, and incubate the mixtures, in the dark, at RT for 1 hr.
- Wash the mixtures with 1.5 ml of PBS three times, and resuspend beads in 0.2 ml of PBS. Load an aliquot (4 µl) into a well on an immunofluorescence microscope slide (Teflon-coated), cover with a cover slip, and observe by fluorescence microscopy using a FITC filter (excitation wavelength: 467-498 nm, emission wavelength: 513-566 nm) and a 10X objective to examine the binding of the fluorescent glycopolymers with the beads14.
Representative Results
Synthesis of glycomonomer
Lactobionic acid was used herein as an example for the preparation of glycomonomers. Using methods in the initial report on the synthesis of LAEMA11, varied yields in the preparation with unsatisfactory purity were observed. The modified purification method using cation and anion exchange resins to remove unreacted starting material offered stable product yield and high purity, which is confirmed by 1H and 13C NMR spectroscopy (Figure 1).
RAFT glycopolymer synthesis and post-modification of glycopolymers with fluorophores
In contrast to the block-glycopolymers prepared through stepped RAFT polymerizations, this one-step copolymerization protocol provides a uniform glycomonomer distribution throughout the polymer backbone. The glycopolymers shown here contain 20 mol% of glycomonomer, 77 mol% of HEAA as a spacer, and 3 mol% of AEMA as a target for post-modifications (see Figure 2). 1H- and 13C-NMR spectroscopy confirmed the structures of PMA-LAEMA and PMA-GAEMA (Figures 3 and 4). As shown in Figure 5, when plotted against the GPC elution profiles of the glycopolymer synthesized without RAFT, both PMA-LAEMA and PMA-GAEMA have low dispersities, proving the efficacy of the RAFT approach. As expected, PMA-GAEMA has a Mn smaller than that of PMA-LAEMA due to PMA-GAEMA’s lack of a pendant sugar. Analysis of the carbohydrates and primary amine functional groups content of the RAFT glycopolymers revealed that the ratio of monomers in the product glycopolymers is consistent with the stoichiometric ratio of starting monomers employed in the RAFT-mediated polymerization reaction (Table 1). This signifies a tight control of the monomer compositions in the synthesized glycopolymers, as designed.
Reaction of primary amine functional groups with activated fluorophores is a widely-used technique in protein labeling. This technique was employed here to label purified glycopolymers with carboxyfluorescein. Following post-modification, fluorescent polymers were obtained (Figure 6). No degradation of the fluorescein-labeled polymers in the reaction was detected by GPC analysis (data not shown).
Binding tests of the synthetic glycopolymers with lectin-coated agarose beads
To assess the lectin-binding specificity of the synthesized glycopolymers, lectin-coated agarose beads with known carbohydrate binding specificity was used. Erythrina crista-galli lectin (ECL), employed in the experiments, has a binding specificity towards β-D-galactoside. Figure 7A clearly demonstrates that PMA-LAEMA-Fluorescein, which contains β-D-galactoside as a pendant carbohydrate, exhibited strong binding with the ECL lectin. In contrast, the negative binding to the ECL of the glycopolymer PMA-GAEMA-Fluorescein, which does not possess a pendant sugar, is shown in Figure 7B. This result exemplifies the binding effectiveness and affinity of the synthesized fluorescent glycopolymer.
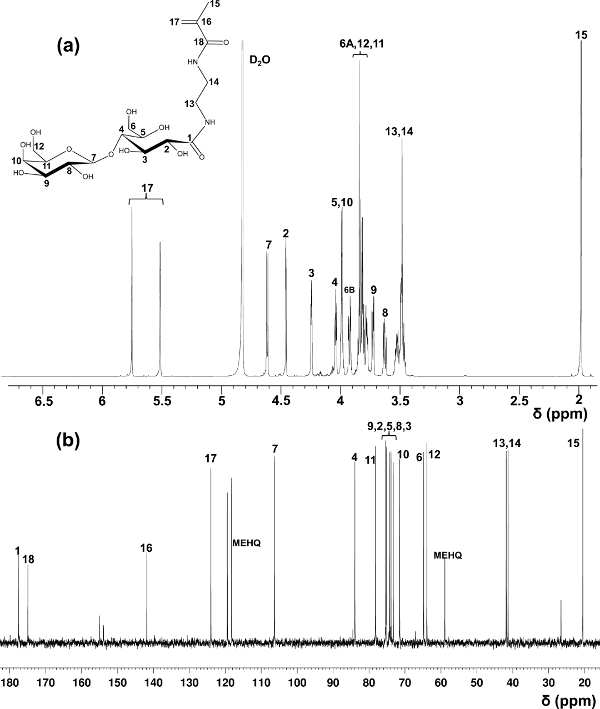
Figure 1. Assigned 1H- (a) and 13C-NMR (b) spectra (D2O) for LAEMA. (This figure has been modified from Wang et al.14) Please click here to view a larger version of this figure.
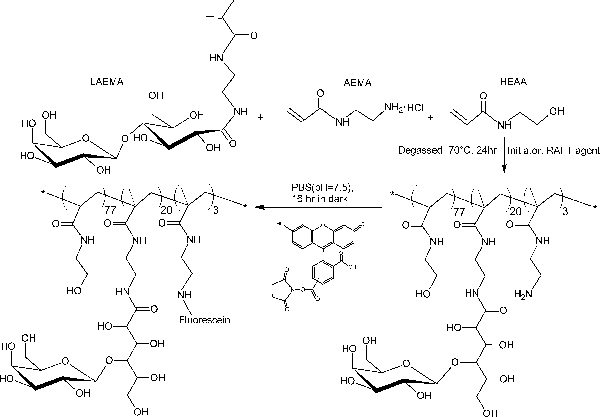
Figure 2. Schematic illustration of the synthesis of fluorescent glycopolymer PMA-LAEMA containing β-galactoside as the pendant sugar. Please click here to view a larger version of this figure.
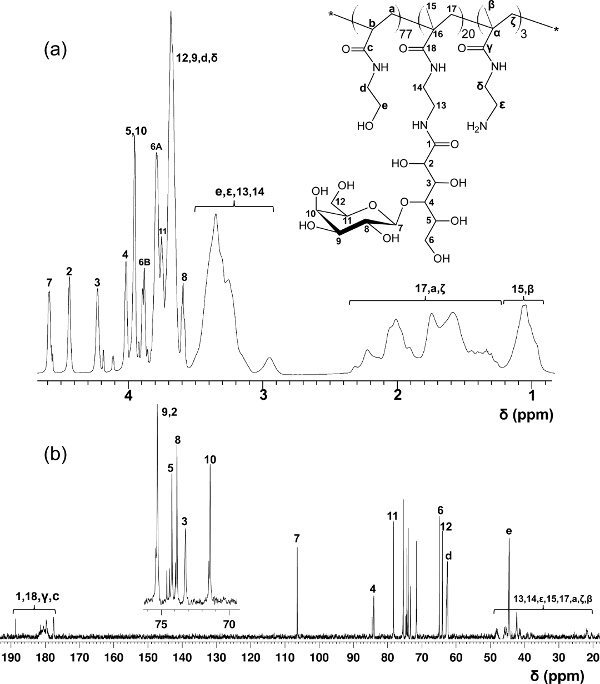
Figure 3. Assigned 1H- (A) and 13C-NMR (B) spectra (D2O) for PMA-LAEMA glycopolymer. (This figure has been modified from Wang et al.14) Please click here to view a larger version of this figure.
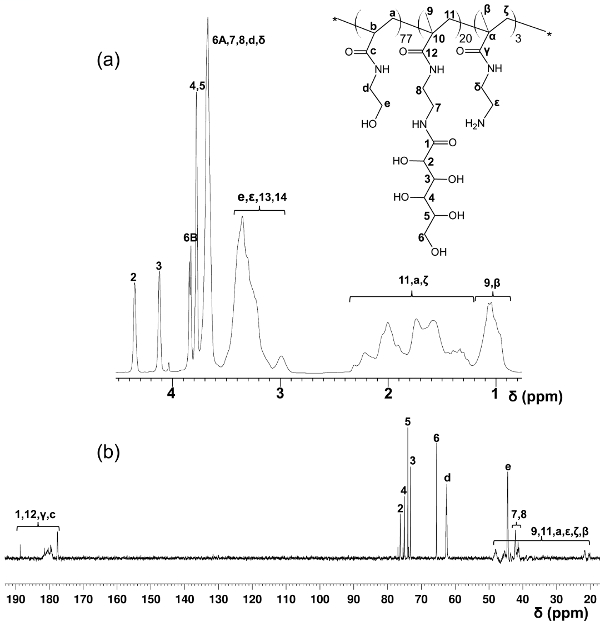
Figure 4. Assigned 1H- (A) and 13C-NMR (B) spectra (D2O) for PMA-GAEMA. (This figure has been modified from Wang et al.14) Please click here to view a larger version of this figure.
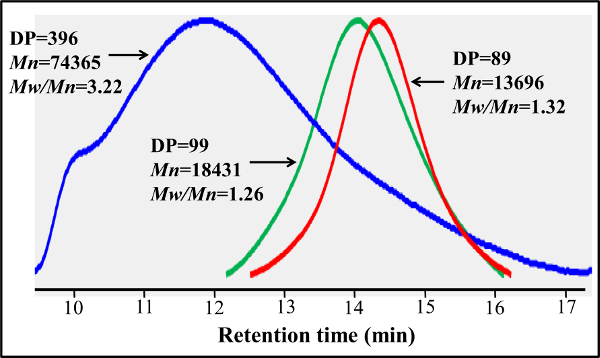
Figure 5. Gel permeation chromatography traces of RAFT-based PMA-GAEMA and PMA-LAEMA prepared with and without using RAFT agent. In contrast to the PMA-LAEMA prepared without RAFT agent (blue), RAFT-based PMA-LAEMA (green) has a much lower dispersity (Mw/Mn). RAFT-based PMA-GAEMA (red) and PMA-LAEMA have similar GPC profiles, but the former has a smaller Mn due to the absence of any pendant sugars. Please click here to view a larger version of this figure.
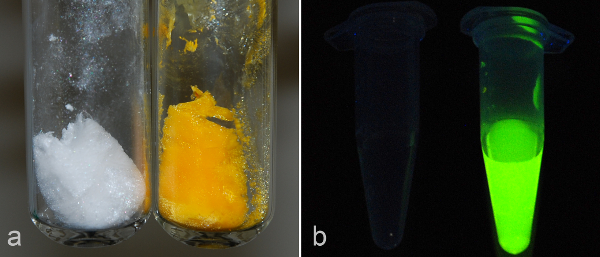
Figure 6. PMA-LAEMA before and after post-modification with fluorophore. (A) Compared with white non-labeled glycopolymer (left tube), fluorescein-labeled PMA-LAEMA shows a strong yellow color (right tube). (B) Under UV, non-labeled PMA-LAEMA (left tube, 1 mg/ml in PBS) is dark and presents with no fluorescence, whereas fluorescein-labeled PMA-LAEMA (right tube, 1 mg/ml in PBS) shows strong green fluorescence. Please click here to view a larger version of this figure.
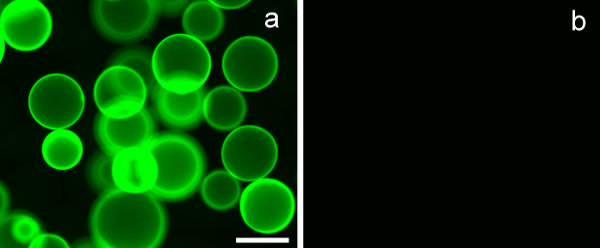
Figure 7. Erythrina crista-galli lectin (ECL)-coated agarose beads bind β-D-galactoside containing glycopolymers, and not those not possessing a pendant sugar. (A) PMA-LAEMA-Fluorescein (3 µg) demonstrated strong binding with ECL, whereas in (B) PMA-GAEMA-Fluorescein, which possesses no pendant β-D-galactoside residue, showed no binding with the lectin-coated beads. Scale bar = 100 µm.
Table 1. Targeting valuesa of synthetic parameters and actual compositions of the glycopolymers. a) Targeting values, values that are desired of the products; b) DP, degree of polymerization; c) NA, not available. Please click here to view a larger version of this figure.
| DPb | Dispersity | Actual content of glycomonomers mol % |
Actual content of primary amine mol % |
|
| Targeting values | 100 | < 1.3 | 20 | 3 |
| PMA-LAEMA | 99 | 1.26 | 19 | 3.2 |
| PMA-GAEMA | 89 | 1.32 | NAc | 2.7 |
Discussion
A facile and efficient protocol for RAFT-based tri-component fluorescent glycopolymers, with and without a pendant carbohydrate, and their use in a lectin-binding test, is demonstrated in this report. The protocol starts with the preparation of glycomonomers LAEMA and GAEMA. Through a one-step RAFT-controlled copolymerization, glycopolymers with reproducible yield, predictable monomer composition and low dispersity, are obtained. Following post-modification of glycopolymers with carboxyfluorescein succinimidyl ester, the binding of the resulting respective fluorescent-labeled glycopolymer is readily testable for its lectin-binding specificity.
In the initial preparative steps of the glycomonomers that are to be employed in the subsequent glycopolymer syntheses, readily available lactobionic acid and gluconolactone were utilized. In theory, any carbohydrates of interest, from monosaccharides to complex oligosaccharides, can be converted to glycomonomers by conjugating the target sugar onto the primary hydroxyl group on C6 of glucose. Following oxidation of the reducing glucose residue, and its subsequent dehydration to a lactone, the product can then be readily reacted with the primary amine on AEMA to form the corresponding glycomonomer. Further examples of this route can be seen in a recent report14. It should be noted that before initiating any polymerization step, MEHQ, a potent polymerization inhibitor, must be removed from all monomer and glycomonomer preparations just prior to use. This is readily accomplished by using the minimum amount of methanol to dissolve the glycomonomer that possesses MEHQ then immediately treat it with acetone at -20 °C to precipitate the inhibitor-free product in high yield.
Essential in any radical polymerization scheme, attention to detail and monomer purities are emphasized. As is typical of a RAFT polymerization system, it consists of a radical source, a RAFT reagent, a monomer and solvent. In this visualized presentation, a single-step RAFT polymerization system is described that focuses on the production of statistical copolymers generated from a reaction mixture possessing three different monomers in an aqueous solution. Two separate RAFT-mediated reactions are presented in which one utilizes a glycomonomer that possesses a pendant, non-reducing carbohydrate terminus (i.e., β-D-galactose), and the other, possessing a polyol with no bound carbohydrate residue. Common to both RAFT-mediated reactions were monomers possessing a singular hydroxyl group that serves as a spacer molecule, and another possessing a free amine for post-modification with an amino-reactive fluorophore.
Since the presence of oxygen in the reaction mixture and environment is detrimental to RAFT-mediated polymerization, its removal to trace levels is readily accomplished via several freeze–evacuate–thaw cycles while maintaining the Schlenk tube reaction vessel under high vacuum.
It should be noted that the molar ratio of different monomers in the reaction can be adjusted as needed. Also, by varying the amount of RAFT agent used, the length of the resulting polymers can be controlled18. However, the molar ratio of the RAFT agent to initiator should always be greater than two to assure the low dispersity of the product. Under these conditions, the evolution of the copolymerization is steady, and the reproducibility of the reaction is very high. That being said, it is unlikely that one obtains a totally uniform distribution of all participating monomers within a statistical copolymer, due to their different polymerization speeds. Characterizing the distribution of different monomers within the polymer is still very challenging.
The post-modification method, presented here, is both simpler and more amenable to the use of a wider selection of fluorescent labels, compared to other protocols applied to label glycopolymers2,11. These would include many of the water-soluble amine-reactive fluorophores, quantum dots, biotins, and others. The binding specificities of the synthesized, labeled glycopolymers are readily verifiable using lectins with known binding affinities. PMA-GAEMA possessing no pendant sugar is an appropriate negative control. Glycopolymers with different fluorescent labels prepared via this route have been successfully used in investigations of lectin-mediated bacterial binding14. As presented, this facile and efficient preparation of statistical fluorescent glycopolymers should provide great potential to a wide variety of glycobiological research.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Experiment Station Chemical Laboratories of the University of Missouri, and by the Cystic Fibrosis Association of Missouri.
Materials
| Reagent | |||
| Lactobionic acid | Sigma-Aldrich | 153516 | |
| D-Gluconolactone | Sigma-Aldrich | G2164 | |
| N-(2-hydroxyethyl) acrylamide (HEAA) | Sigma-Aldrich | 697931 | |
| Orange II sodium salt | Sigma-Aldrich | O8126 | |
| Hydroquinone monomethyl ether (MEHQ) | Sigma-Aldrich | 54050 | Polymerization inhibitor |
| N-(2-aminoethyl) methacrylamide hydrochloride (AEMA) | Polysciences, Inc | 24833-5 | |
| Triethylamine | Fisher Scientific | BP-616 | |
| Anion-exchange resin IRN-78 hydroxide-form, 80 mesh | Sigma-Aldrich | 10343-U | |
| Cation-exchange resin 50Wx8, 200 mesh | Sigma-Aldrich | 217514 | |
| Aluminum oxide, ~150 mesh | Sigma-Aldrich | A1522 | Type WN-6, Neutral, Activity Grade Super I |
| Ninhydrin | Sigma-Aldrich | N4876 | An ethanol solution of 0.2 % ninhydrin was used in the test |
| 4-Cyano-4-(phenylcarbonothioylthio)pentanoic acid | Sigma-Aldrich | 722995 | RAFT agent |
| 4,4′-Azobis(4-cyanovaleric acid) | Sigma-Aldrich | 11588 | Polymerization initiator |
| Carboxyfluorescein succinimidyl ester | Life Technologies | C1157 | |
| Erythrina Cristagalli lectin coated agarose bead | Vector Laboratorie | AL-1143 | |
| Solvent | |||
| dH2O | Produced by Barnstead water purification system, 18 megOhm-cm | ||
| Isopropanol | Fisher Scientific | A461-4 | ACS grade or better |
| Methanol | Fisher Scientific | A454-4 | ACS grade or better |
| Absolute ethanol | Fisher Scientific | BP2818-100 | ACS grade or better |
| Dimethylformamide | Sigma-Aldrich | 22705 | ACS grade or better |
| Acetone | Fisher Scientific | A929-4 | ACS grade or better |
| Equipment | |||
| Dialysis membrane (MWCO: 3,500) | Spectrum Labs | 132720 | |
| Polyethylene glycol analytical standard standard | Sigma-Aldrich | O2393 | |
| Schlenk tube, 1 mL | Quark Glass | Customized | |
| TSK-GEL G4000 PWxl | Tosoh Bioscience | 8022 | Used for GPC analysis of the glycopolymers |
| Empower 3 with GPC/SEC package | Waters Corporation | ||
| Waters Alliance HPLC system | Waters Corporation | Equipped with refractive index detector (Waters 2414) and fluorescence detector (Waters 2475) | |
| Avance III 800 MHz NMR Spectrometer | Brucker Corporation | ||
| BX43 fluorescence microscope | Olympus Corporation | Used with FITC filter in the glycopolymer binding test | |
| Rotavap / Rotoevaporator | Heidolph | ||
| Fritted disc funnel | Fisher Scientific | 10-310-109 | |
| Lyophilizer | Labconco | ||
| Immunofluorescence microscope slide | Polysciences | 18357-1 | |
| Revco Ultima Plus -80C Freezer | Thermo Scientific | ||
| Plastic Vacuum Bag and Hand Pump | Ziploc | ||
| Vacuum Pump, Direct Drive, Maxima C Plus | Fisher Scientific | ||
| Vacuum Gauge | Sargent-Welch | ||
Riferimenti
- Scharfman, A., et al. Pseudomonas aeruginosa binds to neoglycoconjugates bearing mucin carbohydrate determinants and predominantly to sialyl-Lewis x conjugates. Glycobiology. 9 (8), 757-764 (1999).
- Song, E. H., et al. In vivo targeting of alveolar macrophages via RAFT-based glycopolymers. Biomaterials. 33 (28), 6889-6897 (2012).
- Wolfenden, M. L., Cloninger, M. J., Wang, B., Boons, G. .. -. J. Chapter 14. Multivalency in carbohydrate binding. Carbohydrate Recognition: Biological Problems, Methods, and Applications. , 349-370 (2011).
- Moad, G., Rizzardo, E., Thang, S. H. Radical addition-fragmentation chemistry in polymer synthesis. Polymer. 49 (5), 1079-1131 (2007).
- Spain, S. G., Gibson, M. I., Cameron, N. R. Recent advances in the synthesis of well-defined glycopolymers. J. Polym. Sci., Part A: Polym. Chem. 45 (11), 2059-2072 (2007).
- Bernard, J., Hao, X., Davis, T. P., Barner-Kowollik, C., Stenzel, M. H. Synthesis of various glycopolymer architectures via RAFT polymerization: From block copolymers to stars. Biomacromolecules. 7 (1), 232-238 (2006).
- Bulmus, V. RAFT polymerization mediated bioconjugation strategies. Polym. Chem. 2, 1463-1472 (2011).
- Ting, S. R. S., Chen, G., Stenzel, M. H. Synthesis of glycopolymers and their multivalent recognitions with lectins. Polymer Chemistry. 1, 1392-1412 (2010).
- Vazquez-Dorbatt, V., Lee, J., Lin, E. W., Maynard, H. D. Synthesis of glycopolymers by controlled radical polymerization techniques and their applications. Chembiochem. 13, 2478-2487 (2012).
- Jiang, X., Ahmed, M., Deng, Z., Narain, R. Biotinylated glyco-functionalized quantum dots: Synthesis, characterization, and cytotoxicity studies. Bioconjugate Chem. 20 (5), 994-1001 (2009).
- Deng, Z., Li, S., Jiang, X., Narain, R. Well-defined galactose-containing multi-functional copolymers and glyconanoparticles for biomolecular recognition processes. Macromolecules. 42 (17), 6393-6405 (2009).
- Qin, Z., et al. Galactosylated N-2-hydroxypropyl methacrylamide-b-N-3-guanidinopropyl methacrylamide block copolymers as hepatocyte-targeting gene carriers. Bioconjugate Chem. 22 (8), 1503-1512 (2011).
- Albertin, L., Wolnik, A., Ghadban, A., Dubreuil, F. Aqueous RAFT polymerization of N-acryloylmorpholine, synthesis of an ABA triblock glycopolymer and study of its self-association behavior. Macromol. Chem. Phys. 213 (17), 1768-1782 (2012).
- Wang, W., Chance, D. L., Mossine, V. V., Mawhinney, T. P. RAFT-based tri-component fluorescent glycopolymers: synthesis, characterization and application in lectin-mediated bacterial binding study. Glycoconj. J. 31 (2), 133-143 (2014).
- Deng, Z., Ahmed, M., Narain, R. Novel well-defined glycopolymers synthesized via the reversible addition fragmentation chain transfer process in aqueous media. J. Polymer Sci. Part A: Polym. Chem. 47 (2), 614-627 (2009).
- Noel, S., Liberelle, B., Robitaille, L., De Crescenzo, G. Quantification of primary amine groups available for subsequent biofunctionalization of polymer surfaces. Bioconjugate Chem. 22 (8), 1690-1699 (2011).
- Fox, A., Morgan, S. L., Gilbart, J., Biermann, C. J., McGinnis, G. D. Preparation of alditol acetates and their analysis by gas chromatography (GC) and mass spectrometry (MS). Analysis of Carbohydrates by GLC and MS. , 87-170 (1989).
- Thomas, D. B., et al. Kinetics and molecular weight control of the polymerization of acrylamide via RAFT. Macromolecules. 37 (24), 8941-8950 (2004).

