Microelectrode Guided Implantation of Electrodes into the Subthalamic Nucleus of Rats for Long-term Deep Brain Stimulation
Summary
A method for implanting electrodes into the subthalamic nucleus (STN) of rats is described. Better localization of the STN was achieved by using a microrecording system. Furthermore, a stimulation set-up is presented that is characterized by long-lasting connections between the head of the animal and the stimulator.
Abstract
Deep brain stimulation (DBS) is a widely used and effective therapy for several neurologic disorders, such as idiopathic Parkinson’s disease, dystonia or tremor. DBS is based on the delivery of electrical stimuli to specific deep anatomic structures of the central nervous system. However, the mechanisms underlying the effect of DBS remain enigmatic. This has led to an interest in investigating the impact of DBS in animal models, especially in rats. As DBS is a long-term therapy, research should be focused on molecular-genetic changes of neural circuits that occur several weeks after DBS. Long-term DBS in rats is challenging because the rats move around in their cage, which causes problems in keeping in place the wire leading from the head of the animal to the stimulator. Furthermore, target structures for stimulation in the rat brain are small and therefore electrodes cannot easily be placed at the required position. Thus, a set-up for long-lasting stimulation of rats using platinum/iridium electrodes with an impedance of about 1 MΩ was developed for this study. An electrode with these specifications allows for not only adequate stimulation but also recording of deep brain structures to identify the target area for DBS. In our set-up, an electrode with a plug for the wire was embedded in dental cement with four anchoring screws secured onto the skull. The wire from the plug to the stimulator was protected by a stainless-steel spring. A swivel was connected to the circuit to prevent the wire from becoming tangled. Overall, this stimulation set-up offers a high degree of free mobility for the rat and enables the head plug, as well as the wire connection between the plug and the stimulator, to retain long-lasting strength.
Introduction
Deep brain stimulation (DBS) is a treatment based on the delivery of electrical impulses via implanted electrodes to specific cerebral structures, such as the internal globus pallidus1, the subthalamic nucleus (STN)2–4 or the ventral intermediate thalamus5. In the last two decades, this treatment has been established as a powerful therapeutic tool for Parkinson's disease1–4, dystonia6 and tremor7, and is also used to modulate chronic pain7, psychiatric disorders (i.e., obsessive–compulsive disorder8, major depression9) or intractable epilepsy10,11. Furthermore, DBS might, in the future, become a treatment option for refractory arterial hypertension12 or orthostatic hypotension13.
The physiological mechanisms underlying the effects of DBS remain poorly understood. Studies in anesthetized rodents have provided insight into neural responses to high-frequency stimulation that mimic clinically applied DBS14. However, these studies not only lack behavioral corroboration of the DBS effect but also result in considerable variability depending on the stimulation parameters applied14.
To investigate more concisely the behavioral effects and underlying mechanisms of DBS in conscious rodents, a stimulation set-up is needed that fulfills specific requirements. DBS is mostly used as a long-term therapy (e.g., Parkinson's disease, chronic pain). Thus, the stimulation set-up in rodents should be designed so that the unit consists of an electrode with a plug, as well as a wire from the plug to an external stimulator; and this unit should be lightweight but unbreakable when fixed onto the skull. Furthermore, freedom of movement is indispensable for rats during stimulation over a prolonged period. The target structures of DBS are small; for example, the STN in rats has a length of 1.2 mm and a volume of 0.8 mm3,15. Therefore, electrodes must be designed such that the nucleus is not lesioned during insertion and targeting needs to be precise. As most DBS studies conducted in rodents have used landmark based stereotactic insertion of the electrode to the target structure, the error rate can be relatively high, even when using the coordinates according to Paxinos and Watson16. This results in a larger number of animals needed to reach a statistically meaningful result.
In the present study an electrode implantation technique is introduced, that targets the STN with high accuracy by using a microrecording system while advancing the electrode. In addition, a stimulation system is presented that does not only allow a high degree of mobility for the stimulated animal but also guarantees continuous stimulation via secure fixation of the stimulation wire (which is protected by a stainless-steel spring) onto the head of the rat.
Protocol
Animal experiments were approved by the University of Würzburg and the legal state authorities (Lower Franconia, approval number: 54-2531.01-102/13) and performed according to the recommendations for research in experimental stroke studies17 and the current Animal Research: Reporting of In Vivo Experiments Guidelines (http://www.nc3rs.org.uk/arrive-guidelines).
1. Anesthesia
- Check the anesthetic system to ensure adequate amounts of supply gas (oxygen) and isoflurane for the duration of the procedure. Connect the nosecone with the incisor bar of the stereotaxic instrument and put the incisor bar on -3.3 mm.
- Turn on the supply gas (2 L/min). Place the rat into a box and seal the top. Turn on the isoflurane vaporizer to 3.5%.
- When the rat is recumbent, switch the system so that the anesthetic gas flows to the nosecone which is fixed to the incisor bar.
- Remove the rat from the box chamber and shave the area between the ears and the eyes; using a cotton bud soaked with Jodosept PVP, swab the shaved area to remove any loose hair.
- Position the rat in the nosecone (Figure 1) and continue the anesthesia with isoflurane 2.5% in O2 (1 L/min). Check the level of anesthesia by pinching the interdigital area. If the rat is anesthetized adequately, the defensive reflexes are abolished (i.e., withdrawal of the foot).
- Monitor respiration and response to stimulation during procedure and adjust the vaporizer as needed.
- Apply vet ointment on eyes to prevent dryness while under anesthesia. Monitor and maintain body temperature at 37 ± 0.5 °C by a feedback-controlled heating system.
2. Surgery
- Keep the surgical field sterile during the whole surgery. Once the surgeon´s hands are sterile and the operating field is sterile, move only carefully and remember not to break sterility. This includes having also a sterile field (i.e., sterile waterproof drapes) on which one may set down instruments.
- Inject 0.2 ml mepivacaine subcutaneously into the center of the shaved area. Mepivacaine is a local anesthetic that has a duration of action of up to 3 hr. It will further anesthetize the surgical area.
- Using a scalpel, make a midline incision starting between the ears and extending towards 2 cm. Ensure that the periosteum (shiny membrane under the skin) is also incised. Expose the skull with four clamps (Figure 2).
- Using a cotton bud, gently remove the periosteum until the coronal and sagittal sutures are exposed; thereafter, stanch the blood with cotton wool.
- Determine the coordinates of the bregma using a needle fixed at a probe holder, and then mark the tip of the needle with a black felt-tip pen. Using the anterior/posterior (AP), midline/lateral (ML) and dorsoventral (DV) drive screws, position the tip of the needle directly over the bregma.
- Take the AP and ML vernier scale readings: subtract 3.6 mm from the AP reading and 2.5 mm from the ML reading for electrode implantation into the right STN, or add 2.5 mm for electrode implantation into the left STN. This position will be marked by the dye of the felt-tip pen after lowering the tip of the needle to the surface of the skull.
- Clamp the dental drill onto the large probe holder of the stereotaxic instrument. Move the dental drill to the calculated area – i.e., the marked point on the skull. Looking through the microscope, drill a hole (diameter about 1 mm) through the skull until the dura is visible (the skull is about 1 mm thick). Retract the dura using micro-dissection forceps or a sterile needle. The dura is tough enough to destroy the tip of the electrode.
- Drill a hole with the dental drill in each frontal squama, and in the interparietal squama opposite to the electrode hole. Disconnect the probe holder from the stereotaxic instrument. Do not drill on a skull suture as venous vessels follow the sutures under the skull.
- Screw a bone screw into each of the five holes. Avoid threading the screws in too deep. For stainless-steel screws (M1.6), 2–3 turns of the screw will adequately hold the screw without putting pressure on the brain. The number of turns will depend on the pitch of the screw. Clamp the probe holder with the electrode in the micromanipulator (Figure 3).
- Using the AP, ML and DV drive screws, move the probe holder with the electrode until the tip is almost touching the bregma. Note the AP, ML and DV vernier scale readings at the bregma. When the readings are made, raise the electrode a few millimeters to prevent the electrode from scraping the skull during movement. To determine the coordinates of the position where the electrode has to be inserted into the hole, add 3.6 mm to the AP reading and add (or subtract) 2.5 mm to the ML reading.
- Using the AP and ML drive screws, move the electrode to the calculated position. At this point, the electrode tip should be situated directly over the drilled electrode hole. Then, by looking through the microscope, lower the electrode to the level of the dura (Figure 4). This level serves as zero-level in the DV direction. Thereafter, gently insert the tip of the electrode into the brain by looking through the microscope.
- Connect the electrode pin to the connector of the recording system. Put a Faraday cage (or substitute it with aluminum foil) over the rat in the stereotaxic instrument (Figure 5). Ground the stereotaxic instrument with the counterpoise of the room that is being worked in.
- Start the recording system. If available, also use a loudspeaker to obtain an acoustic signal of discharges/salves of single units during advancing the electrode.
- Slowly insert the electrode into the brain by recording the electric activity during advancing the electrode. At a depth of between 7.5 and 8.1 mm from the dura, the specific electric activity of the STN is usually detectable (Figure 6). The typical activity of neurons in the STN is characterized by an irregular firing pattern and a high firing rate (mean frequency: 40.9 ± 12.9 Hz)18.
- During the recording, reduce anesthesia as much as possible (e.g., to 0.8–1.0%); low-anesthetized animals show a clearer electric brain activity.
- Swab away any blood or cerebrospinal fluid that was displaced at the surface of the skull when lowering the electrode.
- Mix up a small amount of dental cement and apply it around the electrode and around four of the five screws using a small spatula (Figure 7). The fifth screw will be used to fix the ground wire of the plug.
- Disconnect the electrode pin from the electrode holder and connector of the recording system when the dental cement is fixed.
- Unscrew the screw that was not fixed by dental cement. Put the plug on the electrode pin. Fix the ground wire of the plug with the fifth screw (Figure 8).
- Mix up dental cement and apply it around the plug. As the cement thickens, mold it around the plug to form a cap. Avoid sharp edges of the dental cement that may harm the animal and remove them during hardening (Figure 9A and B).
- Debride wound edges and close them with a suture at the front and behind the cap. Disinfect the wound edges.
- Connect the head plug to the wire that is fixed on a swivel. Remove the rat from the stereotaxic instrument.
- Apply tramadol (12.5 mg/kg, intraperitoneally) at the end of the intervention and then once daily for 2-3 days. Place the rat in a clean cage with thermal support, fix the swivel on this cage (Figure 10) and inspect it carefully for 1 hr.
- Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
3. Stimulation
- Determine the resistance in the animal before stimulation using an impedance meter.
- Connect the plug of the swivel with a wire and the plugs at the other end of the wire with the current output and the output for the ground wire of the stimulator. Connect the stimulator with a computer in order to program the stimulator.
- Choose the parameters of stimulation in the program; for example, the parameters used in Parkinson’s disease are pulse length: 60 μsec; frequency: 130 Hz. Stimulate the rat with an increasing current amplitude until dyskinesia are recognized. Reduce the electric intensity by 10–20% below the intensity that elicited dyskinesia or until neurologic signs disappear and the animal is comfortable. Monophasic rectangular pulses were used in this study.
- After completing the experiment, euthanize the animal with isoflurane: Adjust the isofurane flow rate or concentration to 5% or greater. Continue isoflurane exposure until 1 min after breathing stops.
Representative Results
Implanting an electrode into the STN of a rat using a recording system – as presented here – is an effective and accurate procedure for DBS that takes approximately 1 hr per animal. This model is a fairly minor procedure: out of 10 rats subjected to surgery, all survived the intervention. Twenty-four hr after intervention, the state of each rat was monitored and no animal achieved more than 1 of 3 points according to the severity code. During the period of continuous stimulation (14 days, 24 hr a day), no wire detached, broke or was bitten through. None of the 10 rats lost the cap of dental cement nor did they get hurt by the equipment during the phase of stimulation. The impedance measured in these 10 animals before stimulation was 353 ± 101 kΩ . Rats were stimulated at a frequency of 130 Hz and a pulse width of 60 µsec. The mean stimulus intensity was 60 µA, which was set at 20% below the intensity threshold of orofacial or contralateral forepaw dyskinesia, thereby preventing problems with feeding or locomotion during the period of stimulation.
Fourteen days after intervention and continuous stimulation, all 10 rats were euthanized by decapitation after deep anesthesia and brains were rapidly harvested. In a rat brain matrix, a 2 mm thick brain block encompassing the STN was cut and immediately frozen at –80 °C. These brain blocks were cut in coronal sections (8 µm thick). Each section was stained with hematoxylin & eosin to visualize the position where the tip of the electrode was located, as well as to detect sings of inflammation or scar tissue due to the electrode. The success rate for localizing the electrode in the STN was 8 of 10 animals. In these 8 rats, the tip of the implanted electrode was situated in the STN, as shown histologically. Figure 11 illustrates the electrode location in the STN. A small lesion developed after continuous stimulation was found in all rats. This lesion was surrounded by a small number of inflammatory cells (Figure 11).
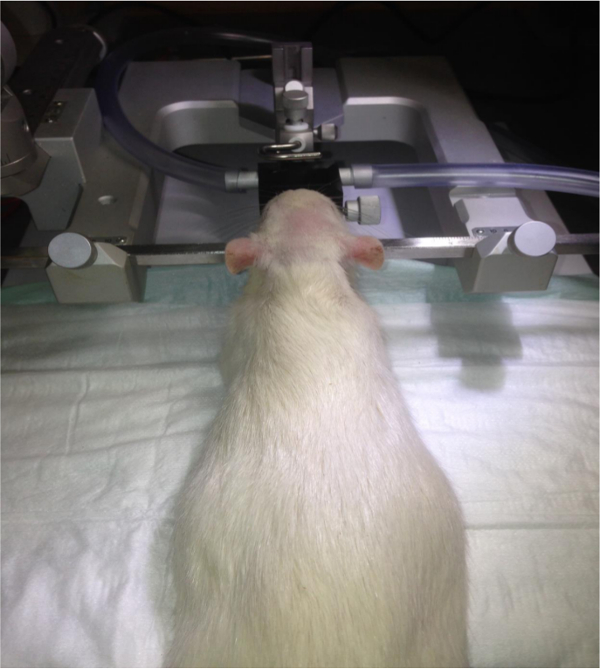
Figure 1. Fixation of the head in the stereotaxic instrument. The rat is fixed by the ear bars of the stereotaxic frame, as well as by the gas anesthesia mask. Please click here to view a larger version of this figure.
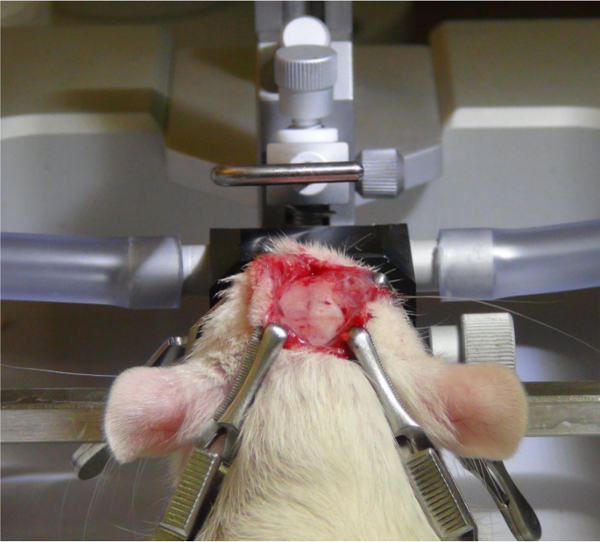
Figure 2. Exposing the skull. After a midline incision, the skin and periosteum are rolled to the wound edges and kept away from the surgical area using four clamps. Please click here to view a larger version of this figure.
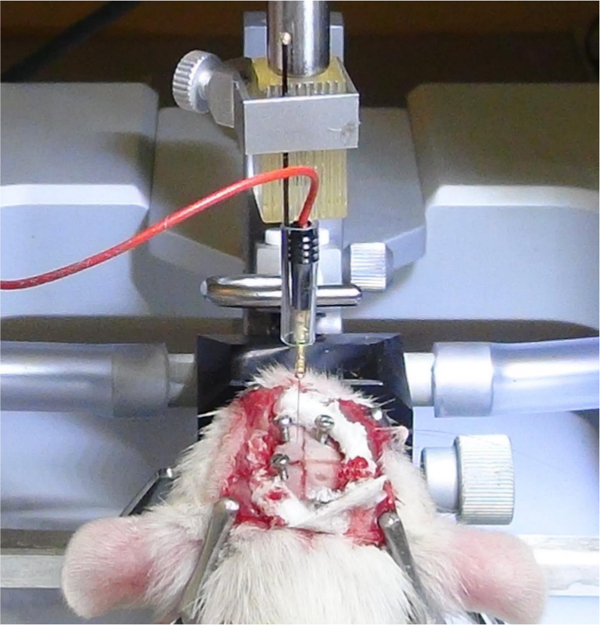
Figure 3. Fixation of the electrode in a probe holder. Using forceps, the pin of the electrode is inserted in the plug and fixed with a probe holder. The plug is connected with the recording apparatus via a wire. Please click here to view a larger version of this figure.
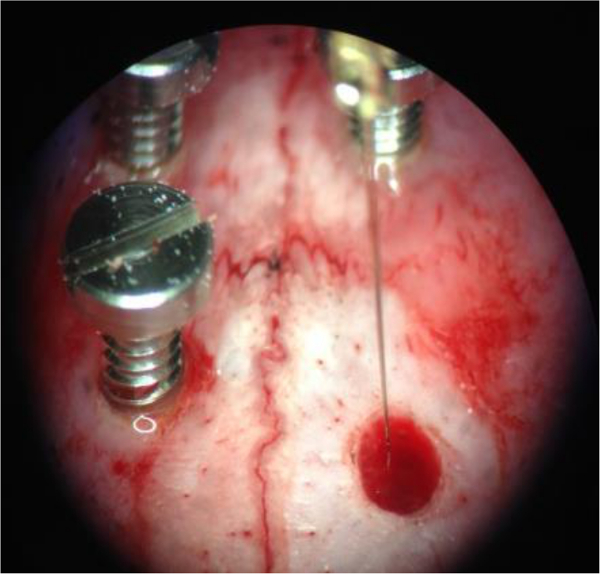
Figure 4. Insertion of the electrode in the brain. After determining the exact AP and ML coordinates of the subthalamic nucleus, the tip of the electrode is advanced to the level of the punctured dura and the dorsoventral vernier scale reading is taken. Please click here to view a larger version of this figure.
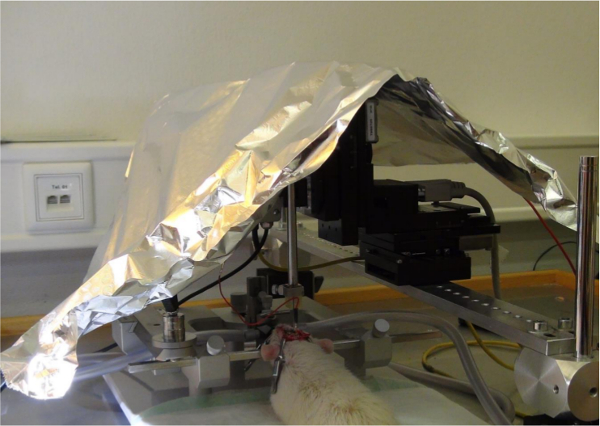
Figure 5. Protection of electric interference. A Faraday cage (or, alternatively, aluminum foil) is put over the rat in the stereotaxic instrument and the instrument, as well as the animal, is grounded. Please click here to view a larger version of this figure.
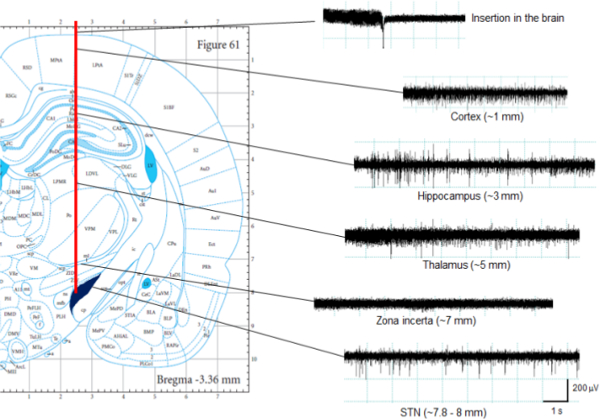
Figure 6. Recording of brain activity. The subthalamic nucleus (STN) shows an irregular firing pattern and a high firing rate (mean frequency: 40.9 ± 12.9 Hz)18. Before entering the STN, the electrode passes a relatively silent region, which is consistent with the zona incerta; the vertical size of this area measures about 0.5–1 mm. Thereafter, the number of spikes increases, indicating that the insertion into the STN is complete. Please click here to view a larger version of this figure.
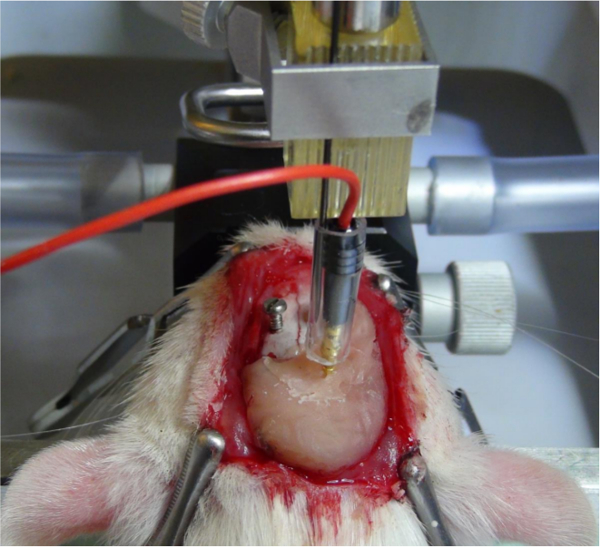
Figure 7. Fixation of the electrode. When the subthalamic nucleus is identified by means of recording, the electrode is fixed by applying dental cement around the electrode shank and the screws. This allows unplugging of the connector from the electrode pin without shifting the position of the electrode. Please click here to view a larger version of this figure.
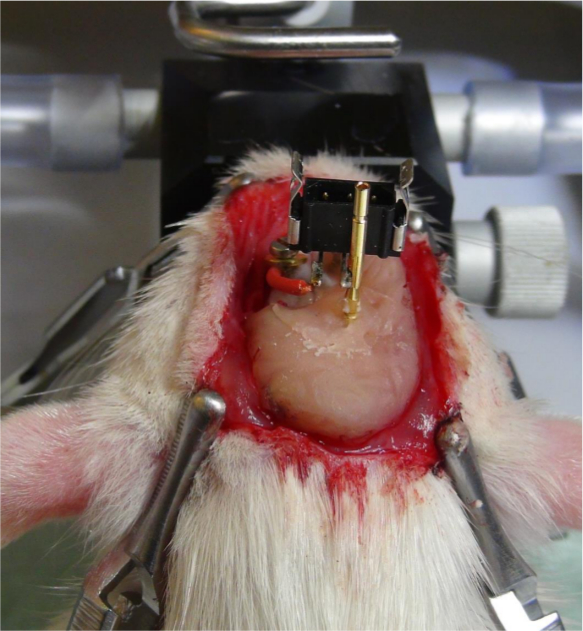
Figure 8. Attaching the plug to the electrode pin. The plug for the connector of the stimulator is attached to the electrode pin. The ground wire, which is soldered onto the plug, is fixed with a screw onto the skull. Please click here to view a larger version of this figure.
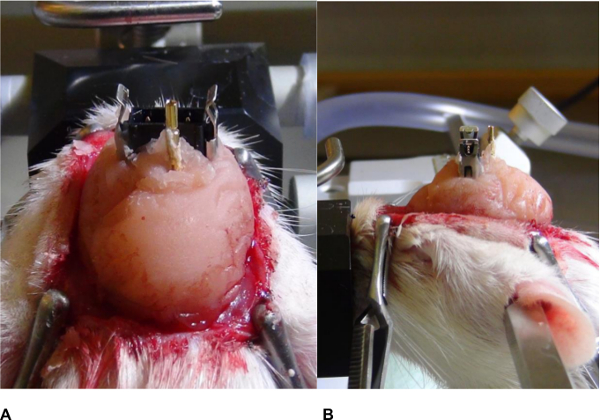
Figure 9. Fixation of the plug. (A) Frontal and (B) Lateral views. Dental cement is applied around the plug and a cap is formed; sharp edges should be avoided. Please click here to view a larger version of this figure.

Figure 10. Connection of the rat to the stimulator. A swivel was joined into the circuit to prevent the wire from becoming tangled. A stainless-steel spring protects the wire if the rat starts to bite the wire. Please click here to view a larger version of this figure.
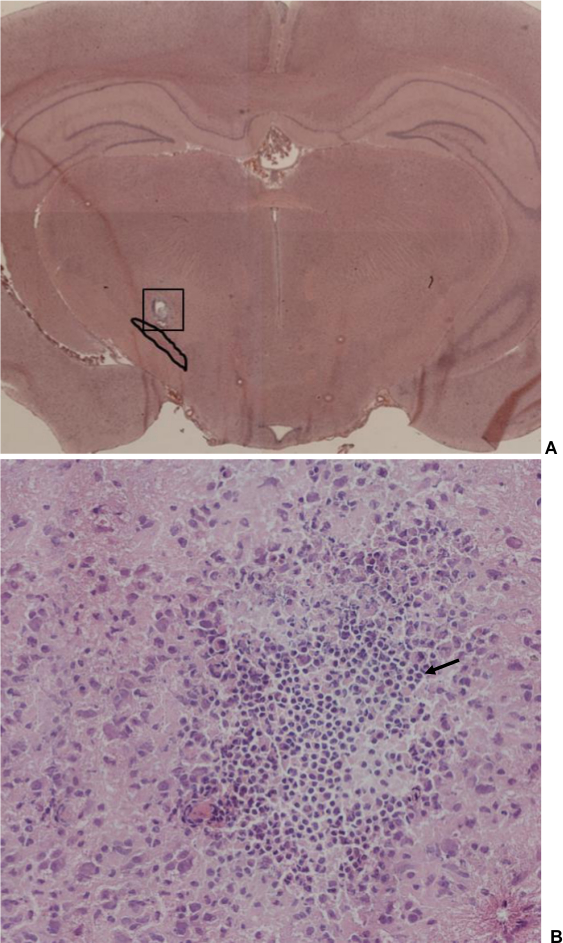
Figure 11. Brain section through the subthalamic nucleus (STN) (hematoxylin & eosin staining). (A) Overview, magnification 2.5. A solid line surrounds the STN. A small lesion is visible where the electrode tip was located during a 14 day period of stimulation. It is of note that there is no penetration canal of the electrode visible (shank diameter: 125 μm), indicating that the electrode is conserving the tissue. (B) Image detail from picture A (box), magnification 100. A small number of inflammatory cells was detectable around the lesion due to the reaction of the brain tissue to the electrode tip. Arrow: indicating an example of an inflammatory cell. Please click here to view a larger version of this figure.
Discussion
This study presents a step-by-step set of instructions for implanting a monopolar chronic electrode into the STN of rats. Although tungsten electrodes with low impedance are often used for DBS18,19, a monopolar electrode made of platinum/iridium (Pt/Ir) was employed that had an impedance of about 1 MΩ. Pt/Ir electrodes are also used in patients with Parkinson’s disease because of their favorable properties: they demonstrate minimal erosion20 and do not produce relevant tissue damage21 if no high-charge densities are used. Since the aim of this study was a long-term stimulation set-up and, in order to achieve a translational approach, electrodes with the aforementioned specifications were applied in the present experiment. Histologic examination of brain slices showing the localization of the electrode tip corroborated the attenuated foreign-body response of Pt/Ir22 in this experiment.
In the present study, electrodes of Pt/Ir with an impedance of 1 MΩ were used. Electrodes with lower, or even higher impedance, are only suitable for either recording cerebral activity or for stimulating cerebral structures, but not both. In contrast, an electrode impedance of 1 MΩ , as used in our study, is suitable for both, recording the activity of deep brain areas and stimulating cerebral structures such as the STN. The major advantage of recording is the identification of the STN location in a short amount of time. Recording allows reliable localization of the STN, as our results have shown: histologic control yielded a high success rate of targeting the STN (8 of 10 animals). The electrode tip was implanted into the upper cell layers of the dorsal-lateral portion of the STN (DV: 7.7 mm) which is known to receive motor inputs mainly from the motor cortex23.
Using a wiring system for DBS may be limited by potential complications such as breaking of wires or a low degree of freedom of movement of animals. However, in our set-up, the wires were connected to swivels, which allowed the animals to move freely. Wireless in vivo stimulating systems (often fixed at the head or implanted into the trunk of the animal) are also limited by the requirement for batteries. As the batteries need to be small, the voltage is therefore low. When using 1 MΩ electrodes, a high voltage is required to achieve the desired stimulus intensity and, in turn, results in larger batteries or frequent replacement of batteries. However, an advantage of the stimulus system used in our study is the large voltage compliance range of the stimulator and the option of constant current stimulation. In this mode the stimulator adjusts the voltage to changes in tissue impedance in order to provide a constant current output at the electrode. An impedance change is expected over the long-term course of DBS with the formation of a stable tissue-electrode interface, e.g., glial encapsulation of the electrode tip22.
In summary, the presented method of electrode implantation is technically simple to perform, reliable, and robust, allowing accurate and safe stimulation of the STN in rats without restricting the freedom of movement or even injuring the animal during the long-term course. With small modifications (e.g., using a plug with additional electrical outputs), this protocol is also applicable by implanting microelectrodes in both STNs or other cerebral structures, long-term recordings or both, stimulation of deep brain structures and recording activity of another cerebral region.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We wish to thank Mr Wabbel for preparing the wires and Mr Tietsch for constructing the plugs and cages according to our plans. This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688). Felix Fluri holds a fellowship of the Interdisziplinäre Zentrum für Klinische Forschung (IZKF), University Clinics Würzburg, Germany.
Materials
| Pt/Ir electrode | FHC Inc. | UE | Custom-made: Specification: UEPSEGSECN1M |
| Plugs | GT Labortechnik (Arnstein/Germany) | Custom-made | |
| Pin header | DISTRELEC | 143-95-324 | single-row, 90° 1×3 datamate, Type M80-8420342 |
| Socket | DISTRELEC | 143-95-621 | single-row,straight 2 mm pole no.1×3 datamate, Type M80-8400342 |
| Stainless steel spring | Plastics ONE | SS0102 | Part-#: .120 X .156 Spring ID (mm): 3.0 Spring OD (mm): 4.0 |
| Dental cement/Paladur | Heraeus Kulzer | 64707938 | Liquid, 500 ml |
| Dental cement/Paladur | Heraeus Kulzer | 64707954 | Powder, rose, 500g |
| Head screw | Hummer & Reiss | V2ADIN84 M1.6×3 | |
| Jodosept PVP | Vetoquinol | 435678/E04 | |
| Mepivacain 1% | AstraZeneca | PZN03338515 | |
| Epinephrine | Sanofi-Aventis | PZN00176118 | |
| Tramadolhydrochloride | Rotexmedica | 38449.00.00 |
Riferimenti
- Kumar, R., Lang, A. E., et al. Deep brain stimulation of the globus pallidus pars interna in advanced Parkinson’s disease. Neurology. 55 (12 Suppl 6), S34-S39 (2000).
- Volkmann, J., Allert, N., Voges, J., Weiss, P. H., Freund, H. -. J., Sturm, V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 56 (4), 548-551 (2001).
- Volkmann, J., Allert, N., Voges, J., Sturm, V., Schnitzler, A., Freund, H. -. J. Long-term results of bilateral pallidal stimulation in Parkinson’s disease. Annals of Neurology. 55 (6), 871-875 (2004).
- Odekerken, V. J., van Laar, T., et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. The Lancet Neurology. 12 (1), 37-44 (2013).
- Benabid, A. L., Pollak, P., et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. The Lancet. 337 (8738), 403-406 (1991).
- Volkmann, J., Wolters, A., et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. The Lancet Neurology. 11 (12), 1029-1038 (2012).
- Nguyen, J. -. P., Nizard, J., Keravel, Y., Lefaucheur, J. -. P. Invasive brain stimulation for the treatment of neuropathic pain. Nature Reviews Neurology. 7 (12), 699-709 (2011).
- Kohl, S., Schönherr, D. M., et al. Deep brain stimulation for treatment-refractory obsessive compulsive disorder: a systematic review. BMC psychiatry. 14, 214 (2014).
- Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Mädler, B., Coenen, V. A. Rapid Effects of Deep Brain Stimulation for Treatment-Resistant Major Depression. Biological Psychiatry. 73 (12), 1204-1212 (2013).
- Fisher, R., Salanova, V., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 51 (5), 899-908 (2010).
- DeGiorgio, C., Heck, C., et al. Vagus nerve stimulation for epilepsy: Randomized comparison of three stimulation paradigms. Neurology. 65 (2), 317-319 (2005).
- Callaghan, E. L., McBryde, F. D., et al. Deep Brain Stimulation for the Treatment of Resistant Hypertension. Current Hypertension Reports. 16 (11), 1-10 (2014).
- Green, A. L. M. R. C. S., Wang, S., Owen, S. L. F., Paterson, D. J. D. P., Stein, J. F. D., Aziz, T. Z. D. M. Controlling the Heart Via the Brain: A Potential New Therapy for Orthostatic Hypotension. [Miscellaneous Article]. Neurosurgery June 2006. 58 (6), 1176-1183 (2006).
- Chang, J. -. Y., Shi, L. -. H., Luo, F., Zhang, W. -. M., Woodward, D. J. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson’s disease. Neuroscience, & Biobehavioral Reviews. 32 (3), 352-366 (2008).
- Hardman, C. D., Henderson, J. M., Finkelstein, D. I., Horne, M. K., Paxinos, G., Halliday, G. M. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: Volume and neuronal number for the output, internal relay, and striatal modulating nuclei. The Journal of Comparative Neurology. 445 (3), 238-255 (2002).
- Paxinos, G., Watson, C. H. . The rat brain in stereotaxic coordinates. , (2007).
- Dirnagl, U. Bench to bedside: the quest for quality in experimental stroke research. Journal of Cerebral Blood Flow, & Metabolism. 26 (12), 1465-1478 (2006).
- Maesawa, S., Kaneoke, Y., et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. Journal of Neurosurgery. 100 (4), 679-687 (2004).
- Spieles-Engemann, A. L., Behbehani, M. M., et al. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiology of disease. 39 (1), 105-115 (2010).
- Agnew, W. F., Yuen, T. G. H., McCreery, D. B., Bullara, L. A. Histopathologic evaluation of prolonged intracortical electrical stimulation. Experimental Neurology. 92 (1), 162-185 (1986).
- Harnack, D., Winter, C., Meissner, W., Reum, T., Kupsch, A., Morgenstern, R. The effects of electrode material, charge density and stimulation duration on the safety of high-frequency stimulation of the subthalamic nucleus in rats. Journal of Neuroscience Methods. 138 (1-2), 207-216 (2004).
- Groothuis, J., Ramsey, N. F., Ramakers, G. M. J., van der Plasse, G. Physiological Challenges for Intracortical Electrodes. Brain Stimulation. 7 (1), 1-6 (2014).
- Li, Q., Ke, Y., et al. Therapeutic Deep Brain Stimulation in Parkinsonian Rats Directly Influences Motor Cortex. Neuron. 76 (5), 1030-1041 (2012).

