Cooling Rate Dependent Ellipsometry Measurements to Determine the Dynamics of Thin Glassy Films
Summary
Here, we present a protocol for cooling rate dependent ellipsometry experiments, which can determine the glass transition temperature (Tg), average dynamics, fragility and the expansion coefficient of the super-cooled liquid and glass for a variety of glassy materials.
Abstract
This report aims to fully describe the experimental technique of using ellipsometry for cooling rate dependent Tg (CR-Tg) experiments. These measurements are simple high-throughput characterization experiments, which can determine the glass transition temperature (Tg), average dynamics, fragility and the expansion coefficient of the super-cooled liquid and glassy states for a variety of glassy materials. This technique allows for these parameters to be measured in a single experiment, while other methods must combine a variety of different techniques to investigate all of these properties. Measurements of dynamics close to Tg are particularly challenging. The advantage of cooling rate dependent Tg measurements over other methods which directly probe bulk and surface relaxation dynamics is that they are relatively quick and simple experiments, which do not utilize fluorophores or other complicated experimental techniques. Furthermore, this technique probes the average dynamics of technologically relevant thin films in temperature and relaxation time (τα) regimes relevant to the glass transition (τα > 100 sec). The limitation to using ellipsometry for cooling rate dependent Tg experiments is that it cannot probe relaxation times relevant to measurements of viscosity (τα << 1 sec). Other cooling rate dependent Tg measurement techniques, however, can extend the CR-Tg method to faster relaxation times. Furthermore, this technique can be used for any glassy system so long as the integrity of the film remains throughout the experiment.
Introduction
The seminal work of Keddie Jones and Corey1 showed that the glass transition temperature (Tg) of ultra-thin polystyrene films decreases with respect to the bulk value at thicknesses lower than 60 nm. Ever since, many experimental studies2-11 have supported the hypothesis that the observed reductions in Tg are caused by a layer of enhanced mobility near the free surface of these films. However, these experiments are indirect measures of a single relaxation time, and thus there is a debate12-18 centered on a direct correlation between average thin film dynamics and the dynamics at the air/polymer interface.
To answer this debate, many studies have directly measured the dynamics of the free surface (τsurface). Nanoparticle embedding,19,20 nanohole relaxation,21 and fluorescence22 studies show that the air/polymer interface has dynamics orders of magnitude faster than the bulk alpha relaxation time (τα) with a much weaker temperature dependence than that of τα. Because of its weak temperature dependence, the τsurface of these films,19-22 and enhanced dynamics of thin polystyrene films,23,24 intersects the bulk alpha relaxation (τα) at a single point T*, which is a few degrees above Tg, and at a τα of ≈ 1 sec. The presence of T* could explain why experiments which probe relaxation times faster than * fail to see any thickness dependence on the Tg of ultra-thin Polystyrene films.13-18 Lastly, while direct measurements of the enhanced mobile layer show that it has a thickness of 4-8 nm,20-22 there is evidence that the propagation length of the dynamics at the air/polymer interface is much larger than the thickness of the mobile surface layer.5,25,26
This report aims to fully describe a protocol for using ellipsometry for cooling rate dependent Tg (CR-Tg) experiments. CR-Tg have been previously used to describe the average dynamics of ultra-thin films of polystyrene.23,24,27,28 Furthermore, This technique was recently used to show a direct correlation between the average dynamics in ultra-thin polystyrene films, and the dynamics at the free surface.23 The advantage of CR-Tg measurements over other types of measurements such as fluorescence, nanoparticle embedding, nanohole relaxation, nanocalorimetry, dielectric spectroscopy, and Brillouin light scattering, studies is that they are relatively quick and simple experiments that do not utilize fluorophores or other complicated experimental techniques. Recent advances in spectroscopic ellipsometry allow this technique to be used to efficiently determine the optical properties of ultra-thin films of polymers and other types of hybrid materials with exceptional accuracy. As such, this technique probes the average dynamics of technologically applicable thin films in temperature and time regimes relevant to the glass transition (T ≤ Tg, τα ≥ 100 sec). Furthermore, this technique will provide information on the expansion coefficients of the glassy and supper cooled liquid states as well as the fragility of the system, which can then be compared with data for bulk films. Lastly, CR- Tg experiments can be used for any glassy system so long as the integrity of the film remains throughout the experiment.
Protocol
1. Film Preparation
- Weigh 0.04 g of polystyrene, and place into a 30 ml vial.
- Weigh 2 g of toluene into the vial. A 2% by weight solution of polystyrene in toluene yields a film of approximately 100 nm.
- Let the solution sit O/N to fully dissolve the polystyrene and let the solutions settle.
- Place a 1 cm x 1 cm Silicon (Si) wafer onto a Spin Coater.
- Spin the wafer at 8,000 rpm for 45 sec. While it is spinning, drop approximately 1 ml of toluene on the spinning wafer.
Note: All steps involving spin coating are performed in a fume hood. - On the now stationary Si wafer, add the solution from step 1.3 drop-wise onto the Si wafer until the entire surface of the Si wafer is covered.
- Before the solution dries on the wafer, spin the Si wafer at 4,000 rpm for 20 sec.
- Determine the thickness of the film using ellipsometry (see step 2).
- If the film is the desired thickness, anneal the film in a vacuum oven at 393 K for 15 hr.
2. Determining Film Thickness
- Place the spun cast film onto the ellipsometer stage and measure the ellipsometric angles Ψ(λ) and Δ(λ) at an incident light angle of 70° with a 1 sec acquisition time and the zone averaging setting turned on.
- Using the ellipsometer software, fit the resulting Ψ (λ) and Δ (λ) data to a three-layer model according to manufacturer's protocol. There are no additional user inputs. The first layer is a substrate layer of Si, the second layer is a native oxide layer with a thickness of 1.5 nm, and the third layer is a Cauchy model (n=A+B/λ2 , k=0), which corresponds to the optical properties of the polystyrene film. In this model, A and B are fit parameters, and n and k are the real and imaginary components of the index of the refraction, respectively.
- For the Cauchy layer, fit the thickness and A and B parameters if the film is above 10 nm. If the film is below 10 nm, only fit A.
Note: This will be discussed further in the Representative results section.
3. Cooling Rate Dependent Tg Measurements
- Coat the surface of the heating element of the variable temperature ellipsometer stage with thermal paste.
- Place the annealed polystyrene film onto the heating element.
- Clamp the film tightly onto the heating element.
- Flow 100% dry Nitrogen gas through the temperature stage at a pressure of <69 KPa.
- Using the temperature stage software, create a temperature profile. This temperature profile begins with a heating ramp to 393 K at 150 K/min. Hold the film at 393K for 20 min.
- Then, alternate cooling ramps to 293 K at rates of 150, 120, 90, 60, 30, 10, 7, 3, and 1 K/min with heating ramps to 393 K at 150 K/min. Place a 5 min temperature hold after each ramp.
- In the ellipsometer software, make a temperature dependent ellipsometry model similar to that in section 2. All three layers are the same, except that the substrate is changed to a temperature dependent Si model.
- In the layer for the temperature dependent Si model, turn on the "Use Ext Temp from Parm Log" Parameter.
- Using laboratory equipment controlling software, have the ellipsometer software read the temperature values from temperature stage.
- Align the ellipsometer such that the signal reaches maximum intensity.
- Under "Edit Hardware Configurations", set the fast acquisition time to 1 sec with high-accuracy zone averaging. Set the normal acquisition time to 3 sec with high-accuracy zone averaging.
- Under the "in situ" tab in the ellipsometer software check the "fast acquisition time mode" box, and press "Start Acquisition". Then, start the temperature profile. Before the 3 K/min cooling ramp, uncheck the fast acquisition time box.
4. Determining Values of Tg
- Export the temperature and thickness profiles into the preferred graphing and analysis software, and separate the temperature and thickness data for all 9 cooling rates.
- In order to account for the effect of zone averaging during acquisition on the temperature, take every temperature value, and average it with the temperature value preceding it, such that T=(Ti + Ti-1)/2, where Ti is a temperature value at a given time, and Ti-1 is the temperature of the preceding time point.
- Plot Thickness vs. Temperature for each cooling rate.
- Perform a linear fit on a portion of the Super Cooled Liquid regime (the high temperature regime with the larger expansion coefficient). This regime will be approximately from 393 K to 380 K.
- Perform a linear fit on a portion of the glassy regime of that same set of data. This regime has a lower expansion coefficient, and will be approximately from 293 K to 340 K.
- Find the intersection point of these two lines. The temperature where these lines intersect is the glass transition temperature.
- Do this for all nine ramps.
5. Analyzing Average Thin Film Dynamics
- For a given film thickness plot Log(Cooling Rate (K/min)) vs. 1/Tg (K-1).
- Compare this indirectly to direct measurements of bulk and surface dynamics by the empirical relation: Cooling Rate * τα = 1000.23,24
Representative Results
Fitting Raw Ellipsometry Data
Polystyrene films are transparent in the wavelength range of the ellipsometer (500-1,600 nm). Thus a Cauchy model is a good model for describing the index of refraction of polystyrene films. Figure 1A shows an example of Ψ(λ) and Δ(λ) for a thick (274 nm) film of polystyrene, and the resulting fit to the Cauchy model 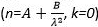 . For films thicker than 10 nm, both the A and B parameters of the Cauchy equation should be fit to accurately model the wavelength dependence of the index of refraction. The Cauchy model is only physical when n is a decreasing function of wavelength, λ. Figure 1B shows an example of a physical index as evident by the always decreasing value of n and k=0. For films thinner than 10 nm, the short path length of light means only the A parameter in the Cauchy equation should be fit. In these extremely thin films, having B as an open fit parameter can drive the ellipsometry fit to an unphysical index, even if the "fit" of Ψ(λ) and Δ(λ) has a small mean squared error (MSE). Such an example can be seen in Figure 2. For some materials it may be necessary to fit higher order terms in the Cauchy model or use a more sophisticated optical model in order to accurately fit optical properties.
. For films thicker than 10 nm, both the A and B parameters of the Cauchy equation should be fit to accurately model the wavelength dependence of the index of refraction. The Cauchy model is only physical when n is a decreasing function of wavelength, λ. Figure 1B shows an example of a physical index as evident by the always decreasing value of n and k=0. For films thinner than 10 nm, the short path length of light means only the A parameter in the Cauchy equation should be fit. In these extremely thin films, having B as an open fit parameter can drive the ellipsometry fit to an unphysical index, even if the "fit" of Ψ(λ) and Δ(λ) has a small mean squared error (MSE). Such an example can be seen in Figure 2. For some materials it may be necessary to fit higher order terms in the Cauchy model or use a more sophisticated optical model in order to accurately fit optical properties.
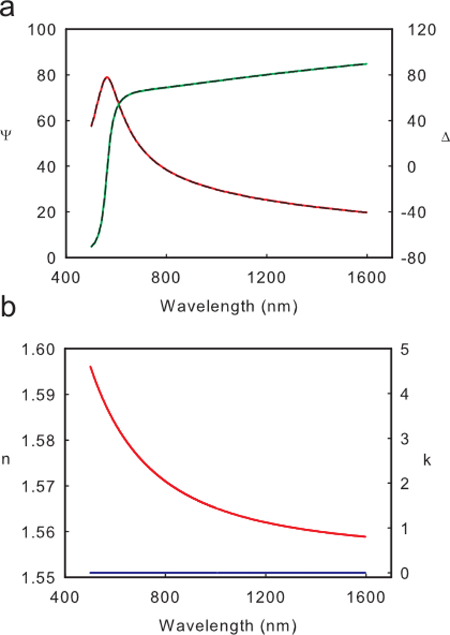
Figure 1. Physical Ellipsometry Fit. (A) An example of Ψ(λ) (red solid line) and Δ(λ) (green solid line) of a 110 nm film of polystyrene, and the resulting fit (black dashed line). (B) An example of the physical index n (red line) and k (blue line) produced by the fit in part A. Please click here to view a larger version of this figure.
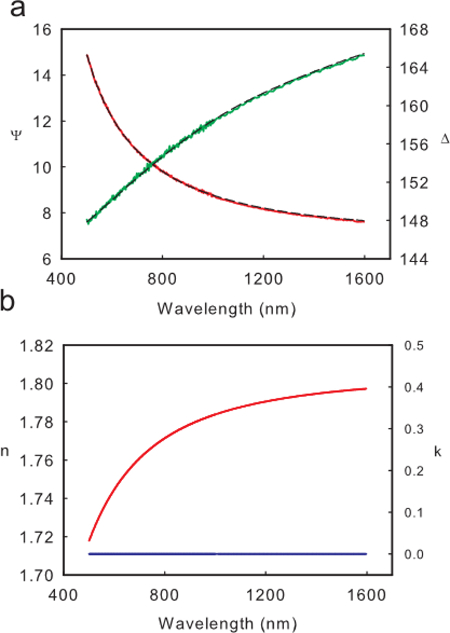
Figure 2. Unphysical Ellipsometry Fit. (A) An example of Ψ(λ) (red solid line) and Δ(λ) (green solid line) of an 8 nm film of polystyrene, and the resulting fit (black dashed line). (B) An example of the unphysical index n (red line) and k (blue line) produced by the fit in part A. Please click here to view a larger version of this figure.
Fitting Cooling Rate Dependent Tg Experiments
When fitting the thickness of a film throughout the temperature profile, it is important to remember both the polystyrene film and the Si wafer substrate will expand, and their optical properties will change with temperature. Thus, in order to calculate accurate expansion coefficients, the index of the Si substrate must be fit with a temperature dependent model to account for changes in the optical properties of Si. An easy way to check to see if the Si substrate is modeled correctly is to see if the fit's MSE changes significantly with temperature. Figure 3A shows an example of a thickness, temperature, and MSE profile for a fit that models the temperature dependence of the index of Si correctly, while Figure 3B shows the same profiles when the fit that does not correctly account for the changes in the optical properties of Si substrate. Notice that the MSE values in Figure 3B vary greatly with temperature. The MSE decrease in Figure 3A is due to switching from an acquisition time of 1 sec to 3 sec.
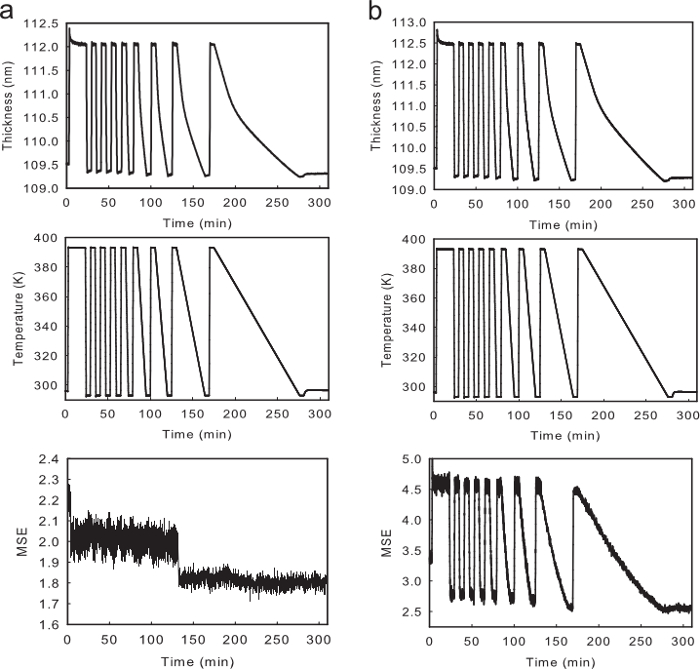
Figure 3. Cooling Rate Tg profiles. (A) An example of a typical temperature, thickness, and MSE profile for a single CR-Tg experiment on a 110 nm polystyrene film when correctly accounting for the temperature dependent index of the Si Substrate. (B) An example of a typical temperature, thickness, and MSE profile for a single CR-Tg experiment on the same film when incorrectly accounting for the temperature dependent index of the Si Substrate. Please click here to view a larger version of this figure.
Assigning Tg
The Tg can be calculated from a thickness vs. temperature plot for a given cooling ramp. Figure 4 shows an example of such a curve. The Tg is defined as the temperature where a supercooled liquid falls out of equilibrium upon cooling. In these ellipsometry experiments, the Tg is defined as the temperature at which linear fits to the supercooled liquid and glassy regimes intersect. Figure 4 highlights these regimes as red and blue, respectively. These regimes should be chosen such that the calculated expansion coefficients agree with previous bulk measurements, if available. This method would eliminate subjectivity from the selection process, which could lead to artificially high or low expansion coefficients, and therefore less accurate measures of Tg. Additionally, the expansion coefficients should be independent of film thickness and cooling rate, which can provide guidance in cases where bulk values of expansion coefficient are not available. The expansion coefficients can be calculated by dividing the slopes of the two regimes by the film thickness. Using this method for determining Tg, the Tg for a 110 nm film of polystyrene is measured to be 372 ± 2 K at 10 K/min, and the expansion coefficients of the supper-cooled liquid and glass are 5.7 x 10-4 ± 3 x 10-5 K-1 and 1.5 x 10-4 ± 3 x 10-5 K-1, respectively, which agree well with previously determined values.29 The errors on the values of Tg, and the expansion coefficients are a result of reasonable changes in the selected regions for the super-cooled and glassy regimes.
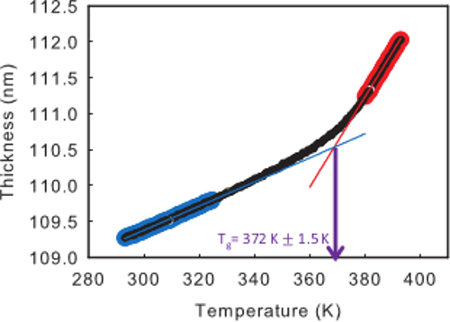
Figure 4. Assigning Tg. A typical plot of thickness vs. temperature for a 110 nm film of 342 kg/mol PS at a cooling rate of 10 K/min. The shaded parts of the curve represent the super-cooled liquid (red) and glassy (blue) regimes chosen for the purposes of assigning Tg. Tg is defined as the temperature at which the two linear fits intersect. Using this method, the Tg for a 110 nm film of polystyrene is measured to be 372 ± 2 K at 1 K/min and the expansion coefficients of the supper-cooled liquid and glass are 5.7 x 10-4 ± 3 x 10-5 K-1 and 1.5 x 10-4 ± 3 x 10-5 K-1, respectively. Please click here to view a larger version of this figure.
Analyzing Average Film Dynamics
The cooling rate dependent Tg data can be related to the average relaxation time at Tg through the empirical relation that at a cooling rate of 10 K/min, the system falls out of equilibrium when the average relaxation time is equal to 100 sec, i.e., cooling rate x τα ≈ 1000.24 Applying this relation to the data in Figure 5A, a plot of log(cooling rate) vs. 1/Tg (Figure 5B) can be used to evaluate how accurate this relation is for polystyrene, and how well the CR-Tg method describes bulk dynamics for a thick film. The red data in Figure 5B are the bulk dynamics of polystyrene as determined via dielectric spectroscopy.16 While the cooling rate x τα ≈ 1000 relation is purely empirical, and may change slightly based on the experimental technique used to determine bulk dynamics, or the specific glass former being tested,30,31 Figure 5B shows that The cooling rate dependent Tg data for a 110 nm film of polystyrene agrees well with this data. This figure also shows that CR-Tg can be used to extend the dynamical range of the measurements to low temperature, which are usually not accessible by dielectric relaxation measurements. Furthermore, the slope of a linear fit of the Log(CR) vs. 1/Tg data is related to the activation energy of the glass transition. This activation energy relates to the fragility (m) of the glassy film at Tg by the relation;
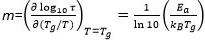
The second term is only correct if an Arrhenius fit to the data is used as an approximation. Using this method, the fragility for a 110 nm PS film is measured to be 162 ± 21. This value is in good agreement with reported values for bulk polystyrene in the literature (150) from dynamic scanning calorimetry measurements.32
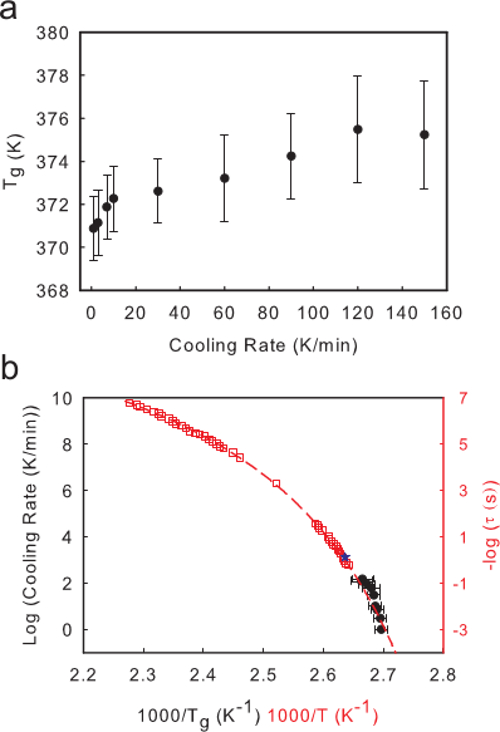
Figure 5. Analyzing Average Film Dynamics via CR-Tg Experiments. (A) A Plot of Tg vs. Cooling Rate for a 110 nm film of polystyrene. (B) Plot of Log (Cooling Rate) vs. 1000/Tg for the same film (black circles). With the relation (cooling rate) x τ = 1000, the results of a CR-Tg experiment on 110 nm PS are plotted alongside direct measures of bulk dynamics of PS, using dielectric relaxation16 with no further shifting factors (red open squares). The red dashed line is a Volgel Fulcher Tammann equation 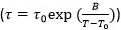 fit to the dielectric relaxation data from reference 16. The resultant fit parameters are τ0 = 1012, B = 13,300 K, and T0 = 332 K. The value of T* from Ref 23 is plotted here as a blue star. From the plot, the fragility is measured to be 162 ± 21. This value is in good agreement with previously reported values in the literature (150).32 Please click here to view a larger version of this figure.
fit to the dielectric relaxation data from reference 16. The resultant fit parameters are τ0 = 1012, B = 13,300 K, and T0 = 332 K. The value of T* from Ref 23 is plotted here as a blue star. From the plot, the fragility is measured to be 162 ± 21. This value is in good agreement with previously reported values in the literature (150).32 Please click here to view a larger version of this figure.
Discussion
Cooling-Rate dependent Tg measurements are high throughput characterization experiments that can determine the Tg, the expansion coefficient of the glass and the super-cooled liquid, the temperature dependence of the average dynamics, and the fragility of a particular glassy material in a single experiment. Furthermore, unlike fluorescence, embedding, or nanohole relaxation experiments, CR-Tg experiments are relatively quick and simple because they do not utilize fluorophores or other complicated experimental techniques. Due to the sensitivity of ellipsometry, this method can be used on films of thicknesses as thin as a few nanometers and as thick as a few microns, so long as the fitting procedure is correct. This allows for quick and simple analysis of both the temperature dependence and thickness dependence of the average dynamics and fragility.
In order to perform these measurements successfully, there are a few critical steps where extra care must be taken. It is imperative that the ellipsometry fit be correct. As explained previously, it is critical that the temperature dependence of the optical properties of the Si substrate be taken into account. Failing to do this could lead to incorrect values of Tg and incorrect values of the expansion coefficient. Also, it is important to clamp the film firmly to the heating element. This helps ensure good thermal contact, which is imperative for accurately defining Tg values at fast rates. Finally, when assigning values of Tg, the chosen supercooled liquid and glassy regimes must not include the glass transition itself. The glass transition is defined as the part of transition where the slope of the thickness vs. temperature data is changing between the supercooled liquid and glassy regimes. Including this change in slope in either linear fit would artificially change the calculate value of Tg. To remove subjectivity from the selection process, choose super-cooled liquid and glassy regimes which produce expansion coefficients that agree with reported values.
Another advantage of this protocol is that it can be amended to allow for the analysis of any glass-former. The only aspect of this protocol that would need to be modified to test the dynamics of a different glass former is the temperature profile. As long as the bulk Tg of the glass former is known, the maximum and minimum temperatures can be altered to ensure that the film undergoes a glass transition, but also doesn't degrade. The maximum temperature should be approximately Tg + 20 K, and the minimum temperature should be at least Tg – 40 K. Also, the chosen cooling rates can be varied to probe other time scales of interest for a particular type of polymer film.
Despite its advantages, there are limitations to this technique. Because this technique indirectly probes an average relaxation time through the cooling rate of the experiment, the time scales this method probes are limited to the maximum cooling rate available by the method of temperature control. For the ellipsometry procedure presented here, the fastest available cooling rate is 150 K/min, which relates to a relaxation time of τ = 6.66 sec. While this time scale is slow enough to be relevant to the glass transition, it is much slower that the time scales relevant to the viscosity of polymer melts. Such time scales usually are determined via rheology or dielectric spectroscopy, but CR-Tg measurements can probe these time scales if the cooling rate is fast enough. This can easily be achieved using nanocalorimetry or flash DSC.33,34
Because of the high-throughput nature of this technique, it allows for many different kinds of materials to be tested. While this report focused on CR-Tg experiments of polystyrene films, this same method could easily be applied to a range of glassy materials from long chain polymers to small organic molecules used in organic electronic technologies. As long as the integrity of the film holds through the experiment, the temperature dependence and thickness dependence of average dynamics and fragility can be determined.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The Authors would like to acknowledge James A. Forrest for help in the initial idea for this technique.26 This work was supported by funding from the University of Pennsylvania and was partially supported by the MRSEC program of the National Science Foundation under award no. DMR-11- 20901 at the University of Pennsylvania.
Materials
| Toluene | Sigma Aldrich | 179418-1L | This can be purchased from any chemical company. |
| Atactic Polystyrene | Polymer Source Inc. | P-4092-S | This can be purchased from any chemical company. |
| THMS 600 temperature stage | Linkam | THMS 600 | any temperature stage that can be fit to an ellipsometer could be used. |
| M2000V Spectroscopic Ellipsometer | J.A. Woollam | M200V | This procedure should be applicable for any spectroscopic ellipsometer. |
| Spin Coater | Laurell Technologies | WS-650-23B | This Procedure is possible with any spin coater |
| Sample vials | Fisher Scientific | 02-912-379 | Any sample vials will do |
| Silicon wafers | Virginia semi conductors | 325S1410694D |
Riferimenti
- Keddie, J. L., Jones, R. A. L., Cory, R. A. Size-Dependent depression of the glass transition temperature in polymer films. Europhys. Lett. 27 (1), 59-64 (1994).
- Forrest, J. A., Veress, K. D., Dutcher, J. R. Interface and chain confinement effects on the glass transition temperature of thin polymer films. Phys. Rev.E. 56 (5), 5705-5716 (1997).
- Forrest, J. A., Mattsson, J. Reductions of the glass transition temperature in thin polymer films: Probing the length scale of cooperative dynamics. Phys. Rev.E. 61 (1), R53-R56 (2000).
- Sharp, J. S., Forrest, J. A. Free surfaces cause reductions in the glass transition temperature of thin polystyrene films. PRL. 91 (23), 235701 (2003).
- Ellison, C. J., Torkelson, J. M. The distribution of glass-transition temperatures in nanoscopically confined glass formers. Nat. Mat. 2 (10), 695-700 (2003).
- Priestley, R. D., Ellison, C. J., Broadbelt, L. J., Torkelson, J. M. Structural relaxation of polymer glasses at surfaces, interfaces, and in between. Science. 309 (5733), 456-459 (2005).
- Ellison, C. J., Kim, S. D., Hall, D. B., Torkelson, J. M. Confinement and processing effects on glass transition temperature and physical aging in ultrathin polymer films: Novel fluorescence measurements. Euro. Phys. J. E. 8 (2), 155-166 (2002).
- Ellison, C. J., Mundra, M. K., Torkelson, J. M. Impacts of polystyrene molecular weight and modification to the repeat unit structure on the glass Transition−Nanoconfinement effect and the cooperativity length scale. Macromolecules. 38 (5), 1767-1778 (2005).
- Yang, Z., Fujii, Y., Lee, F. K., Lam, C. H., Tsui, O. K. C. Glass transition dynamics and surface layer mobility in unentangled polystyrene films. Science. 328 (5986), 1676-1679 (2010).
- Tsui, O. K. C., Zhang, H. F. Effects of chain ends and chain entanglement on the glass transition temperature of polymer thin films. Macromolecules. 34 (26), 9139-9142 (2001).
- Roth, C. B., Dutcher, J. R. Glass Transition and Chain Mobility in thin Polymer Films. J. Electroanal. Chem. 584, 13-22 (2005).
- Ediger, M. D., Forrest, J. A. Dynamics near Free Surfaces and the Glass Transition in Thin Polymer Films: A View to the Future. Macromolecules. 47 (2), 471-478 (2014).
- Serghei, A., Huth, H., Schick, C., Kremer, F. Glassy dynamics in thin polymer layers having a free upper interface. Macromolecules. 41 (10), 3636-3639 (2008).
- Huth, H., Minakov, A. A., Schick, C. Differential AC-chip calorimeter for glass transition measurements in ultrathin films. J. Polym. Sci. B. 44 (20), 2996-3005 (2006).
- Tress, M., et al. Glassy dynamics in condensed isolated polymer chains. Science. 341 (6152), 1371-1374 (2013).
- Boucher, V. M., et al. T g depression and invariant segmental dynamics in polystyrene thin films. Soft Matter. 8 (19), 5119-5122 (2012).
- Yu, M., Olson, E. A., Zhang, M., Zhang, Z., Allen, L. H. Glass transition in ultrathin polymer films: Calorimetric study. PRL. 91 (8), 085703 (2003).
- Kremer, F., Tress, M., Mapesa, E. U. Glassy dynamics and glass transition in nanometric layers and films: A silver lining on the horizon. J. Non-Crys. Solids. 407, 277-283 (2015).
- Qi, D., Ilton, M., Forrest, J. Measuring surface and bulk relaxation in glassy polymers. Euro. Phys. J. E. 34 (6), 1-7 (2011).
- Teichroeb, J. H., Forrest, J. A. Direct imaging of nanoparticle embedding to probe viscoelasticity of polymer surfaces. PRL. 91 (1), 016104 (2003).
- Fakhraai, Z., Forrest, J. A. Measuring the surface dynamics of glassy polymers. Science. 319 (5863), 600-604 (2008).
- Paeng, K., Swallen, S. F., Ediger, M. D. Direct measurement of molecular motion in freestanding polystyrene thin films. J. Am. Chem. Soc. 133 (22), 8444-8447 (2011).
- Glor, E. C., Fakhraai, Z. Facilitation of interfacial dynamics in entagled polymer films. JCP. 141 (9), 194505 (2014).
- Fakhraai, Z., Forrest, J. A. Probing slow dynamics in supported thin polymer films. PRL. 95 (2), 025701 (2005).
- Roth, C. B., McNerny, K. L., Jager, W. F., Torkelson, J. M. Eliminating the enhanced mobility at the free surface of polystyrene: fluorescence studies of the glass transition temperature in thin bilayer films of immiscible polymers. Macromolecules. 40 (7), 2568-2574 (2007).
- Priestley, R. D., Ellison, C. J., Broadbelt, L. J., Torkelson, J. M. Structural relaxation of polymer glasses at surfaces, interfaces, and in between. Science. 309 (5733), 456-459 (2005).
- Gao, S., Koh, Y. P., Simon, S. L. Calorimetric Glass Transition of Single Polystyrene Ultrathin Films. Macromolecules. 46 (92), 562-570 (2013).
- Tropin, T. V., Schulz, G., Schmelzer, J. W. P., Schick, C. Heat capacity measurements and modeling of polystyrene glass transition in a wide range of cooling rates. J. Non-Cryst. Solids. 409, 63-75 (2015).
- Kim, S., Hewlett, S. A., Roth, C. B., Torkelson, J. M. Confinement effects of glass transition temperature, transition breadth, and expansivity: Comparison of ellipsometry and fluorescence measurements on polystyrene films. Eur. Phys. J.E. 30, 83-92 (2009).
- Schawe, J. E. K. Vitrification in a wide cooling rate range: The relations between cooling rate, relaxation time, transition width and fragility. JCP. 141, 184905 (2014).
- Donth, E., Korus, J., Hempel, E., Beiner, M. Comparison of DSC heating rate and HCS frequency at the glass transition. Thermochimica Acta. 304-305, 239-249 (1997).
- Zhang, C., Guo, Y., Priestley, R. D. Confined glassy properties of polymer nanoparticles. J. Polym. Sci. B. 51 (7), 574-586 (2013).
- Koh, Y. P., Grassia, L., Simon, S. L. Structural Recovery of a Single Polystyrene Thin Film Using Nanocalorimetry to Extend the Aging Time and Temperature Range. Thermochimica Acta. 603, 135-141 (2015).
- Gao, S., Simon, S. L. Measurement of the limiting fictive temperature over five decades of cooling and heating rates. Thermochimica Acta. 603, 123-127 (2015).

