Atom Transfer Radical Polymerization of Functionalized Vinyl Monomers Using Perylene as a Visible Light Photocatalyst
Summary
A method for the atom transfer radical polymerization of functionalized vinyl monomers using perylene as a visible-light photocatalyst is described.
Abstract
A standardized technique for atom transfer radical polymerization of vinyl monomers using perylene as a visible-light photocatalyst is presented. The procedure is performed under an inert atmosphere using air- and water-exclusion techniques. The outcome of the polymerization is affected by the ratios of monomer, initiator, and catalyst used as well as the reaction concentration, solvent, and nature of the light source. Temporal control over the polymerization can be exercised by turning the visible light source off and on. Low dispersities of the resultant polymers as well as the ability to chain-extend to form block copolymers suggest control over the polymerization, while chain end-group analysis provides evidence supporting an atom-transfer radical polymerization mechanism.
Introduction
The synthesis of technologically advanced polymers requires precise control over polymer molecular weight, dispersity (Ð), composition, and architecture.1,2 Controlled radical polymerizations (CRPs)3-8 have revolutionized the synthesis of well-defined polymers, with atom transfer radical polymerization (ATRP) being the most used CRP, largely due to operational simplicity and synthetic versatility.9-14 The crux of ATRP is the ability to reversibly deactivate the polymerization, controlling the equilibrium between a propagating radical and a dormant species. Enforcing a low concentration of active radicals greatly minimizes bimolecular termination pathways and allows for the synthesis of well-defined polymers.
Traditional ATRP relies on a transition metal catalyst to mediate this equilibrium.3 These metal catalysts contaminate the polymer product and impede implementation in biomedical or electronic applications while also raising environmental concerns. Although significant strides have been made to reduce the catalyst concentration to ppm levels, these methodologies require more demanding experimental conditions and metal contamination is still not entirely eliminated.15,16
Reversible addition-fragmentation transfer17,18 and nitroxide-mediated polymerizations19,20 are CRPs that do not require metal catalysts, although they have been used less often than ATRP.3 Recently, reversible chain-transfer21 and reversible complexation22,23 variants of ATRP that can use organic catalysts were reported. However, these methodologies require the use of alkyl iodide initiators and are not effective with the alkyl bromides commonly employed in ATRP. A highly desirable CRP would match the performance, feasibility, and robustness of traditional ATRP while being catalyzed by an organic catalyst under mild conditions.
Here, we describe a methodology for the radical polymerization of functionalized vinyl monomers using perylene as a visible-light photocatalyst. Through optimization of parameters such as stoichiometry, concentration, time, and light flux, the molecular weight of the polymers can be controlled.24, 25 Similar methodologies have been recently introduced using phenothiazine derivatives as photocatalysts for metal-free ATRP.26, 27 Because researchers in the field of polymerization catalysis are constantly developing new catalytic systems, the ability to compare catalyst performance across a number of metrics is vital. This ability to make comparisons relies heavily upon procedural consistency and clarity on the part of the researchers performing the experiments. As such, it is our goal that this video will be used to help precisely communicate the methods by which these polymers are synthesized and characterized.
Protocol
CAUTION: Many of the chemicals used in this protocol are hazardous substances. Consult Material Safety Data Sheets (MSDS) and use appropriate personal protective equipment (PPE) when working with these substances.
1. Purification, Preparation, and Storage of Reagents
- Purify all solvents to be used using a solvent purification system according to manufacturer's protocol. If a solvent purification system is not available, use drying agents (e.g., molecular sieves, CaH2, etc.) and distillation. Once dried, store solvents under nitrogen atmosphere in the glovebox at room temperature.
- Purify all monomers by vacuum distillation according to manufacturer's protocol. Once distilled, store monomers in dark bottles under nitrogen atmosphere in a refrigerator.
- Purify initiators by vacuum distillation according to manufacturer's protocol. Once distilled, store initiators in dark bottles under nitrogen atmosphere in a refrigerator.
- Purify the perylene by sublimation according to manufacturer's protocol. Once sublimed, store the perylene on the benchtop at room temperature.
- Prepare a 250 ppm solution of butylated hydroxytoluene (BHT) in deuterated chloroform (CDCl3) by adding 25.0 mg BHT to a 100 g bottle of CDCl3. Prepare and store this solution on the benchtop.
2. Photopolymerization of Methyl Methacrylate Using Perylene as the Photocatalyst
- Allow all reagents to come to room temperature. Inspect all reagents prior to use to ensure there is no sign of contamination, such as discoloration or formation of solid particles.
- In a nitrogen atmosphere glovebox, place a small stir bar in a 20 ml scintillation vial. Add 2.36 mg (9.38 µmol, 1.00 eq.) of perylene.
- Add 1.00 ml dimethylformamide (DMF).
- To this mixture, add 1.00 ml (9.38 mmol, 1,000 eq.) of methyl methacrylate (MMA).
- Place the vial on a stir plate set to 1,600 rpm and illuminated by strips of white light emitting diodes (LEDs). Limit any illumination from other light sources (e.g., overhead lights, nearby windows).
- To initiate the reaction, add 16.4 µl (93.8 µmol, 10.0 eq.) of α-ethyl bromophenyl acetate (EBP) via pipette.
Note: To perform this reaction using natural sunlight, follow the above steps, ignoring step 2.5, then seal the vial, bring it out of the glovebox, and place the vial in an area illuminated by natural sunlight. - Stir the reaction for 24 hr under constant illumination. Isolate and purify the product poly(MMA) by following the instructions in steps 4.1 – 4.4.
3. Kinetic Analysis of the Reaction
- On the benchtop, dispense 0.70 ml of the BHT in CDCl3 solution into a 2 ml vial and seal with septum cap. Bring this vial into the glovebox where the polymerization is being performed.
- Use a syringe to remove 0.20 ml of the reaction mixture. Inject the contents of the syringe into the 2 ml vial containing the 250 ppm solution of BHT in CDCl3. Draw back and push in the plunger several times to ensure thorough quenching of the polymerization.
- Transfer the contents of the 2 ml vial to an NMR (nuclear magnetic resonance) spectroscopy tube. Analyze this sample via 1H NMR spectroscopy for percent conversion.24
- For the specific example of polymerization of methyl methacrylate using perylene, calculate percent conversion from the 1H NMR spectrum of the sample by comparing the area under the peak corresponding to the methoxy hydrogens of the unreacted monomer (δ = 3.62) (M) and the area under the peak corresponding to the methoxy hydrogens of the polymer (δ = 3.50) (P) using the following formula:
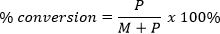
- After analysis, pour the contents of the NMR spectroscopy tube into a clean 20 ml scintillation vial. Evaporate the solvent under reduced pressure. Re-dissolve the sample in 1.00 ml of tetrahydrofuran (THF).
- Send the sample through a syringe filter into a clean 2 ml vial. Analyze the sample via gel permeation chromatography (GPC) coupled with multi-angle light scattering to determine number-average molecular weight (Mn), weight-average molecular weight (Mw), and dispersity (Đ).24
4. Isolation and Purification of the Product Polymer
- Quench the polymerization reaction by pouring the contents of the reaction mixture into a 50-fold excess of methanol and letting stir for at least 1 hr.
- Isolate the poly(methyl methacrylate) from the methanol by vacuum filtration according to manufacturer's protocol.
Note: The isolation method will vary depending on the polymer produced. For poly(methyl methacrylate) and polystyrene, vacuum filter the precipitated polymer from the methanol using a Büchner funnel. For poly(butyl acrylate), decant the methanol from the viscous polymer. - Rinse the polymer with an additional 100 ml methanol.
- Re-dissolve the polymer in dichloromethane and repeat steps 4.1 through 4.3 above twice.
5. Chain-extension of an MMA Macroinitiator with Styrene to Produce Poly(MMA)-b-poly(S)
- Allow all reagents to come to room temperature. Inspect all reagents prior to use to ensure there is no sign of contamination, such as discoloration or formation of solid particles.
- In a nitrogen atmosphere glovebox, place 136 mg (2.34 µmol, 1.00 eq.) of poly(MMA) macroinitiator into a 20 ml scintillation vial fitted with a small stir bar.
- Add 0.59 mg perylene (2.34 µmol, 1.00 eq.).
- Add 1.00 ml DMF.
- Place the vial on a stir plate set to 1,600 rpm and illuminated by strips of white LEDs. Limit any illumination from other light sources (e.g., overhead lights, nearby windows).
- To this mixture, add 1.24 ml (11.7 mmol, 5,000 eq.) of styrene (S) via pipette.
- Stir the reaction for 24 hr under constant illumination. Isolate and purify the product poly(MMA)-b-poly(S) by following the instructions in steps 4.1 – 4.4.
Representative Results
Table 1 shows the range of polymerization results achievable through this method. These data show that perylene is capable of serving as a photocatalyst for the polymerization of a number of functionalized vinyl monomers. For a specific monomer, adjustment of any of a number of reaction parameters such as solvent, stoichiometry, initiator, and light source leads to polymers with varying molecular weights and dispersities ranging from very good to rather broad. Figure 1 shows the results of chain-extension experiments as described in Part 4, and demonstrates that polymers formed using this method are capable of serving as macroinitiators for continued polymerization and formation of block (co)polymers. These results together support the conclusion that, under the correct conditions, perylene facilitates an atom transfer radical polymerization using visible light.
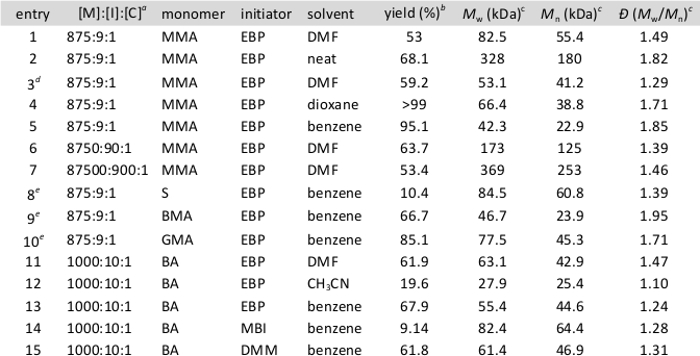
Table 1. Representative results of the polymerization procedure. Unless otherwise noted, polymerizations were performed using 1.00 ml of monomer and 1.00 ml of the solvent specified in the table and run for 24 hours using a white LED as the light source. Monomers used were methyl methacrylate (MMA), glycidyl methacrylate (GMA), butyl acrylate (BA), butyl methacrylate (BMA), and styrene(S). Initiators used were ethyl α-bromophenylacetate (EBP), methyl α-bromoisobutyrate (MBI), and diethyl 2-bromo-2-methylmalonate (DMM). aRatio of monomer to initiator to catalyst (perylene). bIsolated yield. cDetermined using multi-angle light scattering. dConducted for 10 hours using natural sunlight as the light source. ePerformed using 4.00 ml of the specified solvent.
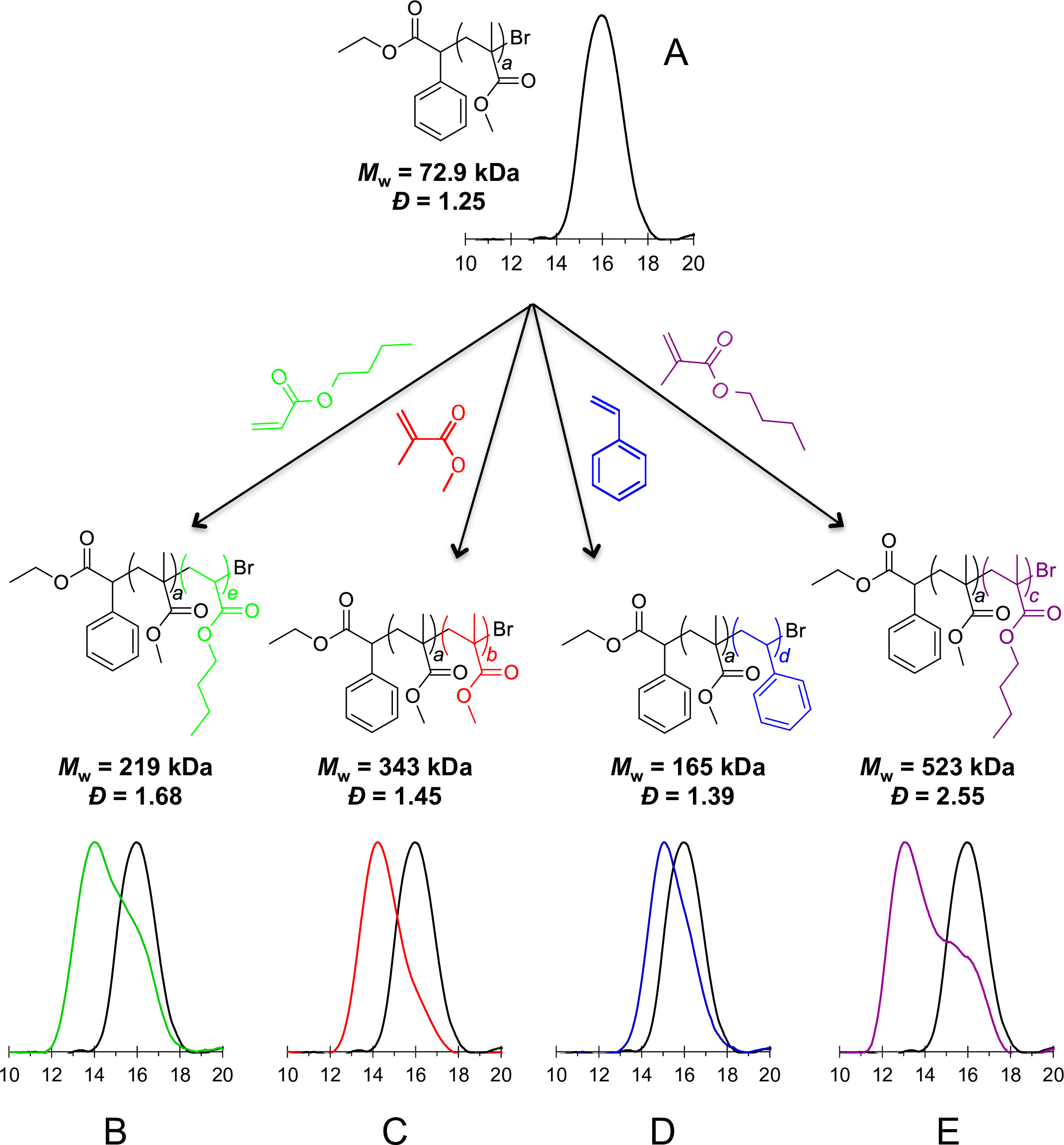
Figure 1. Results of chain-extension polymerizations using a poly(MMA) macroinitiator (A) with butyl acrylate (B), methyl methacrylate (C), styrene (D), and butyl methacrylate (E). Overlaid GPC traces of the poly(MMA) macroinitiator (black) with poly(MMA)-b-poly(MMA) (red), poly(MMA)-b-poly(BMA) (purple), poly(MMA)-b-poly(S) (blue), or poly(MMA)-b-poly(BA) (green).
Discussion
Although the protocol demonstrates a specific example of this polymerization technique, the options available to the researcher performing this reaction are quite broad. Modifications can be made at a number of points throughout the protocol to allow for the optimization of whatever particular photoredox ATRP is being performed. As new monomers, initiators, and catalysts for this reaction come under investigation, the stoichiometry and solvent used to perform the reaction can and should be modified as part of optimization of reaction conditions. Additionally, individual experimenters may choose to use Table 1 as a guideline to modify other parameters such as reaction concentration, light source (whether LED or natural sunlight), and temperature in order to tune the reaction to the exact results desired.
The limitations of this methodology are similar to other, related polymerizations. The reaction is sensitive to oxygen, so rigorous purification of each reaction component is necessary. If the results of this procedure are not found to be consistent, contamination of the reagents is the most likely suspect. Always ensure that all reagents are purified, prepared, and stored as described in Part 1. Additionally, the purification process in Part 3 may need to be modified when synthesizing different polymers according to the solubility profile of the particular polymer. Finally, it is important to note that conventional, uncontrolled radical polymerization can occur if the light flux or temperature is too high. This issue may be indicated by a bimodal molecular weight distribution and/or high values of Đ (> 2.0). It is recommended to use a fan to keep the temperature in the glovebox as close to room temperature as possible. If the light flux is suspected to be too high, it is recommended to either reduce the number of LEDs used or to reduce the voltage supplied to the LEDs. Additional experiments are currently underway to precisely determine the optimal luminous flux range to ensure control over the polymerization.
The results show that perylene is capable of mediating radical polymerization of a number of functionalized vinyl monomers via an oxidative quenching pathway. Control experiments, in which either the catalyst, initiator, or light source was withheld, showed that all three of these components are required for the polymerization to proceed. Additional control experiments also show that the use of air-exclusion techniques (in this case, a glovebox) is necessary, as the presence of oxygen does not allow polymerization to occur. A pulsed-light sequence showed that the polymerization can be halted and resumed by turning the light source off and back on, allowing for temporal control over the reaction. Support for a reversible-deactivation atom transfer mechanism is found through chain extension experiments such as those in Figure 1. When coupled with the relatively low Đ values which can be found in Table 1, there is evidence that this polymerization is an example of photoredox organocatalyzed ATRP, among the first of its kind.
As this new type of ATRP continues to develop and expand, there will be a need to design, test, and optimize many new potential catalysts for the reaction. Such future work will be easiest to interpret when there is consistency and transparency in the methods used to study these reactions. Here, we have communicated the method by which we employ and evaluate organocatalysts for visible light-mediated atom transfer radical polymerization.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the University of Colorado Boulder for its support of this work.
Materials
| perylene, min 98.0% | TCI America | TCP0078-025G | purify by sublimation |
| N,N-dimethylformamide | VWR | EM-DX1726-1 | Omnisolv |
| methyl methacrylate, 99% | VWR | 200000-678 | distilled prior to use, stored in refrigerator |
| ethyl α-bromophenyl acetate | Aldrich | 554065 | distilled prior to use stored in refrigerator |
| butylated hydroxytoluene | Aldrich | W218405 | |
| Chloroform-D | Cambridge Isotope Labs | DLM-7-100 | |
| tetrahydrofuran | VWR | EM-TX0279-1 | Omnisolv |
| methanol | VWR | BDH1135 | |
| dichloromethane | VWR | EM-DX0831-1 | Omnisolv |
| styrene, 99% | VWR | AAAA18481-0F | distilled prior to use, stored in refrigerator |
| glass scintillation vial, 20 mL | VWR | 66022-065 | |
| screw top vial, 2 mL | Agilent | 5182-0715 | |
| septum cap for screw top vial | Agilent | 5182-0717 | |
| heavy wall pressure vessel, 100 mL | Synthware | P160005 | |
| syringe, 1 mL norm-ject | VWR | 89174-491 | |
| NMR tube | New Era | NE-UL5-7' | |
| nylon syringe filter, 0.45 um | VWR | 28143-240 | |
| glovebox | Mbraun | LABstar | |
| solvent purification system | Mbraun | MB-SPS-800 | |
| stirplate | IKA | 3582401 | |
| light-emitting diodes | Creative Lighting Solutions | CL-FRS1210-5M-12V-WH | 2x 12-inch strips of 5500 K white LEDs were used for illumination |
| 12V DC power supply for LEDs | Creative Lighting Solutions | CL-PS16001-40W | |
| high performance liquid chromatograph | Agilent | G1310B, G1322A, G1329B, G1316A | |
| gel permeation size-exclusion columns | Agilent | PL1110-6500 | |
| multi-angle light scattering detector | Wyatt | WTREOS | |
| differential refractometer | Wyatt | WTREX |
Riferimenti
- Bates, F. S., Hillmyer, M. A., Lodge, T. P., Bates, C. M., Delaney, K. T., Fredrickson, G. H. Multiblock Polymers: Panacea or Pandora’s Box. Science. 336 (6080), 434-440 (2012).
- Hawker, C. J., Wooley, K. L. The Convergence of Synthetic Organic and Polymer Chemistries. Science. 309 (5738), 1200-1205 (2005).
- di Lena, F., Matyjaszewski, K. Transition Metal Catalysts for Controlled Radical Polymerization. Prog. Polym. Sci. 35 (8), 959-1021 (2010).
- Rosen, B. M., Percec, V. Single-Electron Transfer and Single-Electron Transfer Degenerative Chain Transfer Living Radical Polymerization. Chem. Rev. 109 (11), 5069-5119 (2009).
- Moad, G., Rizzardo, E., Thang, S. H. Toward Living Radical Polymerization. Acc. Chem. Res. 41 (9), 1133-1142 (2008).
- Braunecker, W. A., Matyjaszewski, K. Controlled/living Radical Polymerization: Features, Developments, and Perspectives. Prog. Polym. Sci. 32 (1), 93-146 (2007).
- Kamigaito, M., Ando, T., Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 101 (12), 3689-3746 (2001).
- Matyjaszewski, K. Comparison and Classifications of Controlled/Living Radical Polymerizations. ACS Symp. Ser. 768 (1), 2-26 (2000).
- Matyjaszewski, K., Tsarevsky, N. V. Macromolecular Engineering by Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 136 (18), 6513-6533 (2013).
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules. 45 (10), 4015-4039 (2012).
- Ouchi, M., Terashima, T., Sawamoto, M. Transition Metal-Catalyzed Living Radical Polymerization: Toward Perfection in Catalysis and Precision Polymer Synthesis. Chem. Rev. 109 (11), 4963-5050 (2009).
- Matyjaszewski, K., Tsarevsky, N. V. Nanostructured Functional Materials Prepared by Atom Transfer Radical Polymerization. Nat. Chem. 1 (4), 276-288 (2009).
- Ouchi, M., Terashima, T., Sawamoto, M. Precision Control of Radical Polymerization via Transition Metal Catalysis: From Dormant Species to Designed Catalysts for Precision Functional Polymers. Acc. Chem. Res. 41 (9), 1120-1132 (2008).
- Matyjaszewski, K., Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 101 (9), 2921-2990 (2001).
- Magenau, A. J. D., Strandwitz, N. C., Gennaro, A., Matyjaszewski, K. Electrochemically Mediated Atom Transfer Radical Polymerization. Science. 332 (6025), 81-84 (2011).
- Matyjaszewski, K., et al. Diminishing Catalyst Concentration in Atom Transfer Radical Polymerization with Reducing Agents. Proc. Natl. Acad. Sci. U.S.A. 103 (42), 15309-15314 (2006).
- Moad, G., Rizzardo, E., Thang, S. H. Living Radical Polymerization by the RAFT Process- A Second Update. Aust. J. Chem. 62 (11), 1402-1472 (2009).
- Moad, G., Rizzardo, E., Thang, S. H. Radical Addition-Fragmentation Chemistry in Polymer Synthesis. Polymer. 49 (5), 1079-1131 (2008).
- Nicolas, J., et al. Nitroxide-Mediated Polymerization. Prog. Polym. Sci. 38 (1), 63-235 (2013).
- Hawker, C. J., Bosman, A. W., Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 101 (12), 3661-3688 (2001).
- Goto, A., Wakada, T., Fukuda, T., Tsujii, Y. A Systematic Kinetic Study in Reversible Chain Transfer Catalyzed Polymerizations (RTCPs) with Germanium, Tin, Phosphorus, and Nitrogen Catalysts. Macromol. Chem. Phys. 211 (5), 594-600 (2010).
- Goto, A., Ohtsuki, A., Ohfuji, H., Tanishima, M., Kaji, H. Reversible Generation of a Carbon-Centered Radical from Alkyl Iodide Using Organic Salts and Their Application as Organic Catalysts in Living Radical Polymerization. J. Am. Chem. Soc. 135 (30), 11131-11139 (2013).
- Goto, A., et al. Reversible Complexation Mediated Living Radical Polymerization (RCMP) Using Organic Catalysts. Macromolecules. 44 (22), 8709-8715 (2011).
- Miyake, G. M., Theriot, J. C. Perylene as an Organic Photocatalyst for the Radical Polymerization of Functionalized Vinyl Monomers Through Oxidative Quenching With Alkyl Bromides and Visible Light. Macromolecules. 47 (23), 8255-8261 (2014).
- Miyake, G. M. Organocatalyzed Photoredox Mediated Polymerization Using Visible Light. US Patent Application. 14, (2013).
- Treat, N. J., et al. Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 136 (45), 16096-16101 (2014).
- Pan, X., Lamson, M., Yan, J., Matyjaszewski, K. Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile. ACS Macro Lett. 4 (2), 192-196 (2015).

