RNA Interference-based Investigation of the Function of Heat Shock Protein 27 during Corneal Epithelial Wound Healing
Summary
Herein, we present a protocol to use heat shock protein 27 (HSP27)-specific small interfering RNA to assess the function of HSP27 during corneal epithelial wound healing. RNA interference is the best method for effectively knocking-down gene expression to investigate protein function in various cell types.
Abstract
Small interfering RNA (siRNA) is among the most widely used RNA interference methods for the short-term silencing of protein-coding genes. siRNA is a synthetic RNA duplex created to specifically target a mRNA transcript to induce its degradation and it has been used to identify novel pathways in various cellular processes. Few reports exist regarding the role of phosphorylated heat shock protein 27 (HSP27) in corneal epithelial wound healing. Herein, cultured human corneal epithelial cells were divided into a scrambled control-siRNA transfected group and a HSP27-specific siRNA-transfected group. Scratch-induced directional wounding assays, and western blotting, and flow cytometry were then performed. We conclude that HSP27 has roles in corneal epithelial wound healing that may involve epithelial cell apoptosis and migration. Here, step-by-step descriptions of sample preparation and the study protocol are provided.
Introduction
Corneal epithelial cells (CECs) are continuously shed into tear film, while they are simultaneously replaced by cells from the limbus and corneal epithelial basal layers.1 Various extrinsic stressors can induce the apoptosis and desquamation of CECs.2 The heat shock proteins (HSPs) are highly conserved and can be divided into two families according to molecular size.3 The largest HSP family includes HSP90, HSP70, and HSP60, and the smaller family includes HSP27.4 The phosphorylation of HSP27 is known to play an important role in cell survival and is required for cell migration because of the role of this protein in actin remodeling.5-7 Therefore, we attempted to test the potential role of HSP27 phosphorylation in CEC migration and apoptosis in an in vitro model of epithelial wound healing.
RNA interference (RNAi) using either small or short interfering RNAs (siRNA) has generated interest in both basic and applied biology, as it potentially allows the expression of any gene of interest to be knocked-down.8 Herein, we used HSP27-specific siRNA to assess the contribution of HSP27 to CEC wound healing and apoptosis. Traditional RNAi methods for gene knock-down in cells use synthetic RNA duplexes, including two unmodified 21-mer oligonucleotides that can be assembled to create siRNAs. The RNAi siRNA that we used in this present study is a simple and highly efficient method to transfect cells, and this reagent works with various immortalized cell lines. In this present study, we demonstrate the methods used for this analysis, including a scratch-induced directional wound assay, western blotting, siRNA transfection assay, immunofluorescence assay, and flow cytometry.
Protocol
1. Cell Line
- Culture 106 telomerase-immortalized human corneal epithelial cells (HCECs) in a 6-well plate (density: 1039.9 cell/mm2) in a 37 °C incubator with a 5% CO2 atmosphere using bronchial epithelium growth medium (BEGM) until they reach 95% confluence.
2. Western Blot Analysis after Creating Epithelial Scratch Wounds
- Streak a sterile 200 µl pipette tip across the surface of a well of confluent cultured HCECs four times per dish in a biological safety cabinet (Class II, Type A2) and incubate HCECs separately in a 37 °C incubator with a 5% CO2 atmosphere for 1, 5, 10, 30, 60, and 120 min.
- Use one 6-well plate for six different samples according to the incubation time after corneal epithelial wounding.
- Wash wounded HCEC monolayers three times with 1x PBS and then add 2.0 ml BEGM to each well.
- Detach HCECs using 2 ml 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) per well for 5 min, centrifuge at 900 x g for 5 min in a 15 ml tube, and aspirate trypsin-EDTA using a 1 ml pipette.
- Suspend HCECs with 1 ml 1x PBS and transfer them to a 1.5 ml tube.
- Centrifuge HCECs at 10,000 x g for 15 sec and aspirate 1x PBS using a 1 ml pipette.
- Resuspend HCECs in 100 µl ice-cold lysis buffer (10 mM Tris, 10 mM NaCl, 2 mM EDTA, 25 mM NaF, 2 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride [PMSF], protease inhibitors [1 µM pepstatin A, 1 µM leupeptin, and 0.1 µM aprotinin] and 0.5% Triton X-100 [pH 7]) and mix them well.
- Incubate cells for 30 min on ice to induce cell lysis.
- Centrifuge lysates at 4 °C at 10,000 x g for 15 min, and then transfer supernatants to fresh 1.5 ml tubes (90 µl aliquots) and store them at -80 °C.
- Determine total protein concentrations of cell lysates using the Bradford protein assay.9
- Load 30 µg total cell proteins into a 10% or 12% acrylamide gel for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then electrophoretically transfer separated protein bands to nitrocellulose filters with a 200 mA current for 1 hr at 4 °C to use in western blot assays.
- Block nitrocellulose filter membranes with 5% skimmed milk in Tris-buffered saline with Tween 20 (TBST) for 1 hr, add primary rabbit polyclonal antibodies against non-phosphorylated HSP27 (1:1,000 dilution) or primary rabbit polyclonal antibody against phosphorylated HSP27 (1:1,000 dilution) in 5% bovine serum albumin (BSA), and incubate the membranes overnight at 4 °C on a shaker.
- Detect immunoreactive bands using horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:10,000 dilution) in 5% BSA after washing for 10 min 3 times with TBST.
- Incubate the membrane in the western blotting luminol reagents (6-7 ml per 10 cm × 5 cm membrane) for 1 min at room temperature.
- Remove the membrane from reagent solution, remove excess liquid with an absorbent towel, and place in a plastic sheet protector.
- Working in a dark room with a safe light, place covered membrane in a film cassette with protein side facing up.
- Place X-ray film on top of membrane and expose for 1 min.
3. siRNA Transfection Assay10
- Culture HCECs at 5×105 cells/well in a 6-well plate in a 37 °C incubator with a 5% CO2 atmosphere using BEGM until they reach 95% confluence.
- Dilute the transfection reagent (2.5 or 7.5 µl) with 100 µl reduced serum medium for transfection (the dilution factor was 41 or 14.3) and dissolve the HSP27-specific and scrambled control siRNA in 100 µl reduced serum medium to create 10 or 50 nM of HSP27-specific and scrambled control siRNA.
- Mix 100 µl siRNA solution with 100 µl diluted transfection reagent (1:1 ratio) and incubate the mixture for 15 min at room temperature.
- Add the siRNA-lipid complexes to cells. Then after 4 hr change media to complete BEGM and incubate the cells for 2 days at 37 °C.
- Analyze transfected cells by western blotting, as described from sections 4.1 to 4.7.
4. Western Blot Assay for siRNA-transfected Cells11
- Extract HSP27-specific and scrambled control siRNA-transfected HCECs in a biological safety cabinet using 100 µl ice-cold lysis buffer (10 mM Tris, 10 mM NaCl, 2 mM ethylenediaminetetraacetic acid (EDTA), 25 mM NaF, 2 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride (PMSF), protease inhibitors, and 0.5% Triton X-100, pH 7).
- Incubate cells for 30 min on ice to induce cell lysis.
- Pellet lysates at 10,000 x g for 15 min and then transfer supernatants to fresh 1.5 ml tubes (90 µl aliquots) and store them at -80 °C.
- Determine protein concentrations of the cell lysates using the Bradford protein assay.9
- Load samples with equal amounts of total cell proteins on a 10% or 12% acrylamide gel, subject the gel to SDS-PAGE, and electrophoretically transfer the separated protein bands to nitrocellulose filters with a 200 mA current for 1 hr at 4 °C to use in western blot assays.
- Block nitrocellulose filter membranes with 5% skimmed milk in Tris-buffered saline with Tween 20 (TBST) for 1 hr, add primary antibodies against phosphorylated and non-phosphorylated HSP27 (1:1,000 dilution), phosphorylated Akt (1:1,000 dilution), non-phosphorylated Akt (1:1,000 dilution, used as a cell survival marker), Bcl-2-associated X protein (1:1,000 dilution, used as a pro-apoptotic protein), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:200 dilution, used as a loading control) in 5% bovine serum albumin (BSA), and incubate the membranes overnight at 4 °C on a shaker.
- Detect immunoreactive bands using horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:10,000 dilution) in 5% BSA after washing 3 times with TBST, 10 min each wash.
- Incubate the membrane in the western blotting luminol reagents (6-7 ml per 10 cm × 5 cm membrane) for 1 min at room temperature.
- Remove the membrane from reagent solution, remove excess liquid with an absorbent towel, and place in a plastic sheet protector.
- Working in a dark room with a safe light, place the covered membrane in a film cassette with the protein side facing up.
- Place X-ray film on top of the membrane and expose for 1 min.
5. Scratch-induced Directional Wounding Assay Evaluation of Cell Migration12
- In a biological safety cabinet, make a wound by dragging a sterile pipette tip across the surface of a well of confluent cultures of HSP27-specific siRNA-transfected or scrambled control siRNA-transfected HCECs.
- Immediately after wounding, wash the cells twice with 1x phosphate buffered saline (PBS) and maintain them in BEGM cultures in a 37 °C incubator with a 5% CO2 atmosphere for 24 hr after wounding.
- Photograph HCEC images using an upright microscope at 100X magnification 24 hr after wounding and perform background flattening using the Filter command in image analysis software.
- Using the Select Measurements command, define the Area of Interest (AOI) with the same sized polygonal shape which can cover perpendicularly from end to end of initial wound, and determine three different AOI in the wounded area of each sample.
- Automatically count the cell number in each field using the Count/Size Measure menu options.
6. Flow Cytometry Analysis of Apoptosis
- Culture HSP27-specific siRNA-transfected and control siRNA-transfected HCECs containing each 10 nM siRNA at a concentration of 5×105 cells/well in 6-well plates until the cells reach 95% confluence in a 37 °C incubator with a 5% CO2 atmosphere in BEGM.
- Detach HCECs using 2 ml 0.25% trypsin-EDTA per well for 5 min, centrifuge at 900 x g for 5 min in a 15 ml tube, and aspirate trypsin-EDTA using a 1 ml pipette.
- Wash cells twice with cold PBS and then resuspend the cells in 1x binding buffer (0.1 M HEPES/NaOH [pH 7.4], 1.4 M NaCl, and 25 mM CaCl2) at a concentration of 106 cells/ml.
- Transfer 100 µl cell suspension (1 x 105 cells) into a 5 ml culture tube.
- Add 5 µl fluorescein isothiocyanate-conjugated Annexin V and 5 µl propidium iodide.
- Gently vortex the cells and incubate them for 15 min at room temperature in the dark.
- Add 200 µl of 1x binding buffer to each tube and analyze the cells by a flow cytometer within 1 hr.
Representative Results
The expression of phosphorylated HSP27 significantly increased at 5, 10, and 30 min after scratch wounding compared with unwounded HCECs13. Western blot analysis revealed that the expression of phosphorylated HSP27 and phosphorylated Akt were both significantly reduced, whereas the expression of Bax was significantly increased in HSP27-specific siRNA-transfected HCECs (Figure 1A-E). The phosphorylated HSP27 expression was reduced by 30% and 40% in 10 nM and 50 nM of HSP27-specific siRNA-transfected cells, respectively, compared with control siRNA-transfected cells, but the phosphorylated HSP27 expression was not reduced (Figure 1A-B). Moreover, the non-phosphorylated HSP27 expression was reduced by 20% and 30% in 10 nM and 50 nM of HSP27-specific siRNA transfected cells, respectively, but the non-phosphorylated HSP27 expression was not reduced (Figure 1A and C).
The scratch-induced directional wounding assay indicated that at 24 hr after wounding, HSP27-specific siRNA-transfected cells at 10 and 50 nM exhibited reduced migration (Figure 2). Moreover, HSP27-specific siRNA-transfected HCECs underwent more apoptotic and necrotic cell death compared with scrambled control siRNA-transfected cells by flow cytometry (Figure 3).
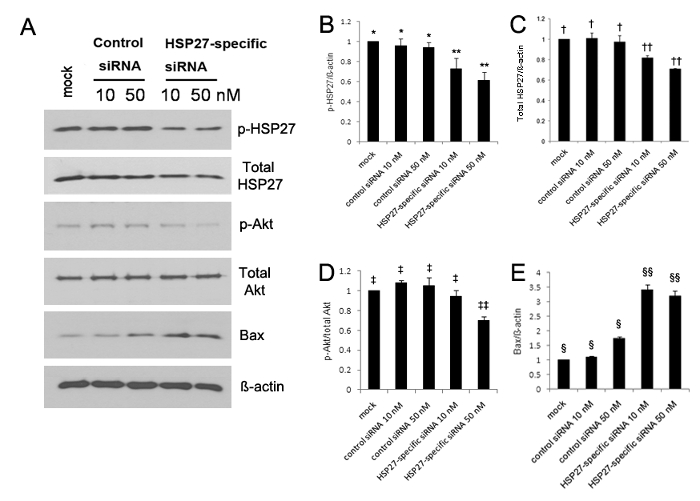
Figure 1. Western blot analysis using antibodies against phosphorylated HSP27 (p-HSP27), non-phosphorylated HSP27 (non-p-HSP27), phosphorylated Akt (p-Akt) as a cell-survival marker, non-phosphorylated Akt (non-p-Akt), Bcl-2eassociated X protein (Bax) as a pro-apoptotic protein, and GAPDH (A). The expression of phosphorylated and nonphosphorylated HSP27 and phosphorylated Akt significantly decreased (B–D), however, the expression of Bax significantly increased in the HSP27-specific siRNA-transfected HCECs (E), compared with that observed in the control siRNA-transfected cells (all p < 0.05). The phosphorylated HSP27 expression was reduced by 30% and 40% in 10 nM and 50 nM of HSP27-specific siRNA-transfected cells compared with mock control, respectively, but the phosphorylated HSP27 expression was not reduced in 10 nM and 50 nM of control siRNA-transfected cells (B). **, *; †, ††; ‡, ‡‡; §, §§: a statistically significant difference among groups (p < 0.05). The error bars represent standard deviation (SD). Please click here to view a larger version of this figure.
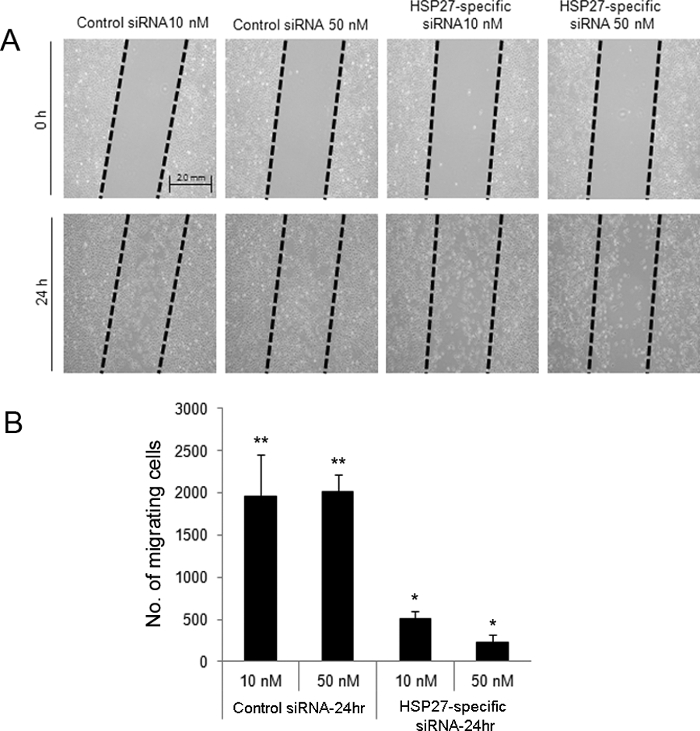
Figure 2. Scratch-induced directional wounding assay to evaluate cell migration after wounding in siRNA-transfected HCECs. A scratch wound was created in control and HSP27-specific siRNA-transfected cells (A). Cells were removed from the 'dragged' areas. At 24 hr after wounding, 10 and 50 nM of HSP27-specific siRNA-transfected cells exhibited lower numbers of migrating cells compared with the 10 and 50 nM control siRNA-transfected cells (B). ** and * indicate a statistically significant difference among groups (p<0.05). The data are shown as means ± standard deviations. Please click here to view a larger version of this figure.
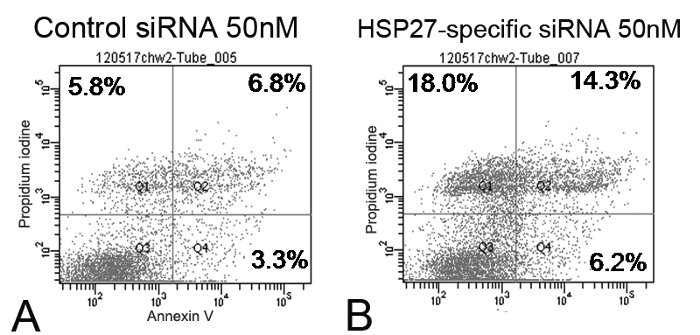
Figure 3. Flow cytometry of 50 nM of scrambled control siRNA and HSP27-specific siRNA-transfected human corneal epithelial cells (HCECs) labeled with annexin V and PI (A and B). The percentage of the total cells in quadrants corresponded to early apoptotic cells (annexin V-positive and PI-negative cells, Q4, lower right), late apoptotic cells (annexin V-positive and PI-positive cells, Q2, upper right), and necrotic cells (annexin V-negative and PI-positive cells, Q1, upper left). HSP27-specific siRNA-transfect HCECs had more apoptotic and necrotic cell death than control siRNA-transfected cells. Please click here to view a larger version of this figure.
Discussion
In this present study, we evaluated the potential role of HSP27 in corneal epithelial wounding using in vitro approaches. The critical steps involved siRNA transfection for HSP27 knock-down to observe the function of HSP27 in cells subjected to stress. Notably, a role for HSP27 was revealed by these experiments in epithelial cell migration and apoptosis during corneal epithelial wound healing. Unlike previous studies10 that used rat HSP27-specific siRNA to transfect vascular smooth muscle cells, we used a siRNA transfection technique to modify gene expression in human CECs to effectively knock-down HSP27-specific gene expression and study HSP27 function. Although there were differences in the target sequence that we used as well as in the cell density, final siRNA concentration, and incubation time, the protocol recommended by the manufacturer was explicitly followed. In terms of alternative methods, HSP27 knock-out mouse may be used to show if HSP27 phosphorylation involves epithelial migration and cell apoptosis. However, it is difficult to monitor the change of HSP27 phosphorylation in mouse model, because its phosphorylation occurs in very short period during epithelial wound healing.
There were several limitations to the present study. First, the in vitro environment in which we cultured human CECs certainly differed from the in vivo environment for human CECs, especially regarding cell survival. Second, the siRNA used in this study was not specific to the phosphorylated form of HSP27 as it affected the overall expression levels of HSP27, including both phosphorylated and non-phosphorylated forms.
In the future, a clinical application of these procedures would be to apply HSP27 to live human wounded corneas. We hope that the current findings will help to advance treatments of corneal epithelial tissue damage.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Student Research Grant (13-14) of University of Ulsan College of Medicine, Seoul, Korea and a grant (2014-464) from the Asan Institute for Life Sciences, Seoul, Korea.
Materials
| Biological safety cabinet | CHC LAB Co.Ltd, Daejeon, Republic of Korea | CHC-777A2-06 | Class II, Type A2 |
| Stealth RNAi™ siRNA | Thermo Fisher Scientific, Inc., Waltham, MA | RNAi siRNA; scrambled control-siRNA and HSP27-specific siRNA | |
| BEGMTM | Lonza, Inc., Walkersville, MD | CC-3171, CC4175 | Bronchial epithelium growth medium |
| Protease inhibitor | Sigma-Aldrich, Inc., St. Louis, MO | P8340 ,P7626 | 1 uM Pepstatin A, 1 uM Leupetin, 0.1 uM Aprotin |
| Bradford protein assay | Bio-Rad Laboratories, Hercules, CA | #500-0001 | Bradford protein assay |
| Nitrocellulose filters | Amersham, Little Chalfont, UK | RPN3032D | Western blotting membrane |
| Non-phosphorylated HSP27 | Abcam Inc., Cambridge, MA | ab12351 | 1:1000 dilution (Total HSP27) |
| Phosphorylated HSP27 (Ser85) | Abcam Inc., Cambridge, MA | ab5594 | 1: 1000 dilution HSP27 was phosphorylated at Ser85 |
| Lipofectamine® RNAiMAX reagent | Invitrogen, Carlsbad, CA | 13-778-075 | Transfection reagent |
| Phosphorylated Akt (Ser473) | Cell Signaling Technology, Danvers, MA | No. 4060 | 1: 1000 dilution Akt was phosphorylated at Ser473 (cell survival marker) |
| Non-phosphorylated Akt | Cell Signaling Technology, Danvers, MA | No. 4061 | 1:1000 dilution (Total Akt) |
| Bcl-2-associated X protein | Cell Signaling Technology, Danvers, MA | No. 4062 | 1: 1000 (anti-apoptotic protein marker) |
| GAPDH | Santa Cruz Biotechnology, Santa Cruz, CA | No. 4063 | 1:1000 loading control marker (house keeping gene) |
| Horseradish peroxidase-conjugated goat anti-rabbit antibodies | Thermo Fisher Scientific, Inc., Waltham, MA | NCI1460KR | 1:10000 dilution |
| OPTI-MEM | Invitrogen, Carlsbad, CA | 31985 | reduced serum medium for transfection |
| Image analysis software | Olympus, Inc., Tokyo, Japan | Image-Pro Plus 5.0 | |
| Skimed milk powder | Carl Roth GmbH + Co. KG, Karlstruhe, Germany | T145.2 | |
| Tris | Amresco LCC, Inc. Solon, OH | No-0497 | |
| Sodium Chloride | Amresco LCC, Inc. Solon, OH | No-0241 | |
| Six well culture plate | Thermo Fisher Scientific, Inc., Waltham, MA | 140675 | 35.00 mm diameter / well |
| 24-well culuture dish | Thermo Fisher Scientific, Inc., Waltham, MA | 142475 | |
| Orbital shaker | N-Bioteck, Inc., Seoul, South Korea | NB1015 | |
| Bovine serum albumin | Santa Cruz Biotechnology, Santa Cruz, CA | sc-2323 | |
| BDFACSCantoTM II | BD Biosciences, Franklin Lakes, NJ | Flow cytometry | |
| X-Ray Film | Kodak, Rochester, NY | Medical X-Ray Cassette with Green 400 Screen | |
| western blotting luminol reagent | Santa Cruz Biotechnology, Santa Cruz, CA | sc-2048 | |
| FITC Annexin V Apoptosis Detection Kit I | BD Biosciences, Franklin Lakes, NJ | 556547 |
Riferimenti
- Dua, H. S., Gomes, J. A., Singh, A. Corneal epithelial wound healing. Br. J. Ophthalmol. 78 (5), 401-408 (1994).
- Estil, S., Primo, E. J., Wilson, G. Apoptosis in shed human corneal cells. Invest. Ophthalmol. Vis. Sci. 41 (11), 3360-3364 (2000).
- Guay, J., et al. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J. cell. Sci. 110, 357-368 (1997).
- Park, J. W., et al. Differential expression of heat shock protein mRNAs under in vivo glutathione depletion in the mouse retina. Neurosci. Lett. 413 (3), 260-264 (2007).
- Rane, M. J., et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J. Biol. Chem. 278 (30), 27828-27835 (2003).
- Shin, K. D., et al. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J. Biol. Chem. 280 (50), 41439-41448 (2005).
- Jain, S., et al. Expression of phosphorylated heat shock protein 27 during corneal epithelial wound healing. Cornea. 31 (7), 820-827 (2012).
- Alekseev, O. M., Richardson, R. T., Alekseev, O., O’Rand, M. G. Analysis of gene expression profiles in HeLa cells in response to overexpression or siRNA-mediated depletion of NASP. Reprod. Biol. Endocrinol. 7, 45 (2009).
- Park, H. Y., Kim, J. H., Lee, K. M., Park, C. K. Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and corea. Exp. Eye. Res. 94 (1), 13-21 (2012).
- Voegeli, T. S., Currie, R. W. siRNA knocks down Hsp27 and increases angiotensin II-induced phosphorylated NF-kappaB p65 levels in aortic smooth muscle cells. Inflamm. Res. 58 (6), 336-343 (2009).
- Shi, B., Isseroff, R. R. Arsenite pre-conditioning reduces UVB-induced apoptosis in corneal epithelial cells through the anti-apoptotic activity of 27 kDa heat shock protein (HSP27). J. Cell. Physiol. 206 (2), 301-308 (2006).
- Shen, E. P., et al. Comparison of corneal epitheliotrophic capacity among different human blood-derived preparations. Cornea. 30 (2), 208-214 (2011).
- Song, I. S., et al. Heat shock protein 27 phosphorylation is involved in epithelial cell apoptosis as well as epithelial migration during corneal epithelial wound healing. Exp Eye Res. 118 (1), 36-41 (2014).

