Affinity Labeling Detection of Endogenous Receptors from Zebrafish Embryos
Summary
A novel technique for the detection of low abundance endogenous receptors present in zebrafish embryos is described. We have named it AFLIP because it consists of affinity labeling of the receptor by its ligand linked to immunoprecipitation.
Abstract
By combining the powers of Affinity Labeling and Immunoprecipitation (AFLIP), a technique for the detection of low abundance receptors in zebrafish embryos has been implemented. This technique takes advantage of the selectivity and sensitivity conferred by affinity labeling of a given receptor by its ligand with the specificity of the immunoprecipitation. We have used AFLIP to detect the type III TGF-β receptor (TGFBR3), also know as betaglycan, during early zebrafish development. AFLIP was instrumental in validating the efficacy of a TGFBR3 morphant zebrafish phenotype. In the first step, embryo protein extracts are prepared and used to generate 125I-TGF-β2-TGFBR3 complexes that are purified by immunoprecipitation. Later, these complexes are covalently cross-linked and revealed using SDS-PAGE separation and autoradiography detection. This technique requires the availability of a labeled ligand for, and a specific antibody against, the receptor to be detected, and shall be easily adapted to identify any growth factor or cytokine receptor that meets these requirements.
Introduction
Specific detection of proteins expressed during embryonic development is required to validate expression profiles obtained by measuring their cognate mRNAs with RT-PCR or in situ hybridization (ISH). This is commonly achieved by a western blot of embryo extracts followed by detection with specific antibodies. However, this approach is hard to apply to proteins that are in very low abundance, or that have properties that hamper their quantitative transfer during their blotting. Betaglycan, also known as the type III transforming growth factor β (TGF-β) receptor (TGFBR3), is an example of these difficulties. TGFBR3 is a part time membrane proteoglycan that binds TGF-β through its core protein1, with notably higher affinity for the isoform TGF-β2, a property that distinguishes it from any other TGF-β binding protein2. TGFBR3 in the zebrafish is expressed from 8 hpf on, reaching a maximum by 72 hpf, as detected by RT-PCR of its mRNA3.
However, despite the availability of a very specific antibody3, every attempt to detect its translated product by western blot proved unsuccessful. Reckoning that TGFBR3’s proteoglycan nature, as well as putative low abundance may be accountable for this failure, a detection method, AFLIP, which takes advantage of TGFBR3 high affinity for TGF-β2 was devised. In this method a protein extract from pooled embryos is allowed to specifically bind 125I-labeled TGF-β2 and the receptor-ligand complexes are purified by immunoprecipitation and cross-linked before separation by SDS-PAGE. The migration patterns observed by autoradiography of the gels revealed the presence and nature of the labeled receptor species. This approach combines the ligand specificity of affinity labeling with immunoprecipitation by specific antibodies, increasing detection range, avoiding the inefficient transfer blotting of TGFBR3. Due to its inherent properties, the AFLIP assay is not a quantitative assay but can be used to confidently gauge relative experimental differences in the analyzed receptor.
Protocol
All experiments carried out in animals were approved by the Committee for Laboratory Animal Care and Use of the Autonomous National University of México (UNAM), under the CICUAL-Protocol number: FLC40-14. (CICUAL: "Comité Institucional para el Cuidado y Uso de los Animales de Laboratorio del Instituto de Fisiologìa Celular, Universidad Nacional Autònoma de México").
1. Preparation of Embryo Protein Extract
- Collect 100 – 200 embryos for each condition to compare (morphants vs. wild type) at desired stage, for example 72 hr post fertilization (hpf).
- Place embryos in Petri dishes containing fish water (see Table 1) and manually dechorionate embryos using fine-tipped forceps. Avoid using pronase during this step (or any other protease throughout the protocol) as it may digest the targeted receptor.
- Place embryos in a 1.5 ml microfuge tube and wash embryos twice with 1x phosphate buffer solution (PBS) (see Table 1).
- Add 500 µl of deyolk buffer (see Table 1).
- Release most of yolk by gently pipetting up and down (about 30 – 40 times) the embryos in the solution. For 72 hpf and 48 hpf embryos use blue and yellow pipette tips, respectively. Yellow tips may need to be slightly cut to avoid destroying embryos. The successful yolk release can be checked by observing embryos under microscope.
- Collect embryos by centrifugation at 600 x g for 15 sec.
- Discard supernatant carefully by using a pipet.
- Wash embryos twice with 500 µl washing buffer (see Table 1) by gently vortexing at lowest speed.
- Collect embryos by centrifugation at 600 x g for 15 sec.
- Starting from here, continue the procedure at 4 °C.
- Discard supernatant carefully by using a pipet.
- Resuspend embryos in 350 µl of lysis buffer (see Table 1) and homogenize using a plastic pestle.
- Incubate lysed embryos 30 min with agitation on a test tube rocker at 4 °C.
- Centrifuge lysed embryos at 11,000 x g for 15 min in order to remove insoluble debris.
- Transfer cleared supernatant using a pipet to a fresh tube.
- Determine total protein by Bradford protein assay4, or other suitable procedure. If Bradford assay is used, perform the calibration curve in the presence of equivalent concentrations of the detergents presents in the lysis buffer, as they cause an underestimation of the protein standard4.
2. Detection of Endogenous Receptor Protein
- Affinity Labeling and Immunoprecipitation (all these steps at 4 oC)
- Place 400 – 500 µg of total embryo protein in a 1.5 ml microfuge tube and dilute to 1 µg/µl with buffer 1 (see Table 1).
Note: In order to obtain 500 µg of total embryo protein, about 100 – 200 embryos must be initially processed. 72 hpf embryos routinely yield ~ 5 µg of total embryo protein, which is sufficient for betaglycan detection. - Add enough volume of labeled TGF-β2 stock to reach a final concentration of 150 pM and incubate with agitation on a test tube rocker 2 hr at 4 °C. TGF-β2 must be labeled before AFLIP is started, with 125I by the chloramine T method as described by Cheifetz et al.5.
CAUTION: Use shielding to minimize exposure while handling 125I labeled ligand. - Add enough volume of undiluted antibody against the receptor of interest in order to reach a 1:100 dilution and continue incubation for another 2 hr at 4 °C (incubation may be O/N). This is the optimal dilution of antiserum # 31, used in this study and described elsewhere3, but depending on the quality of the antibody, a larger or smaller dilution may be the optimal.
- Add 50 µl of suitable immunoglobulin binding beads (e.g., G-protein-Sepharose, which was previously equilibrated in TNTE and resuspended 1:5 of its original volume in TNTE) and incubate for 50 min with agitation on a test tube rocker at 4 °C.
- Recover the beads by microcentrifugation at 11,000 x g for 20 sec.
- Discard supernatant in an appropriate radioactive trash container.
- Wash the IP-beads three times with 1 ml of IP wash buffer (see Table 1), by vortexing and microcentrifugation at 11,000 x g for 20 sec each time.
- Resuspend IP-beads in 250 µl of IP wash buffer.
- Add 1.5 µl of disuccinimidyl suberate (DSS, dissolved in DMSO at 10 mg/ml). Prepare the DSS solution just before its use in this step.
- Incubate for 15 min at 4 °C with agitation.
- In order to quench the crosslinking reaction, add 500 µl of IP wash buffer, supplemented with enough Tris-Cl pH 7.4 stock to reach 25 mM Tris-Cl. The free amino groups in Tris capture unreacted DSS.
- Centrifuge at 11,000 x g for 20 sec to collect IP-beads and discard supernatant.
- Resuspend IP-beads in 30 µl of reducing Laemmli buffer.
- Boil samples 5 min at 94 °C.
- Optionally, analyze samples in a gamma counter using manufacturer's protocol.
- Place 400 – 500 µg of total embryo protein in a 1.5 ml microfuge tube and dilute to 1 µg/µl with buffer 1 (see Table 1).
- Sample Analysis
- Subject samples to denaturing SDS-PAGE. Use polyacrylamide at the appropriate percentage for the mass of the receptor and run gel under standard procedure.
- Immerse gel in fixative solution (see Table 1) for 30 min at RT.
- Wash gel with distillated water for 15 min.
- Place gel in previously hydrated filter paper and cover with saran wrap film.
- Dry gel at 80 °C for 1 hr.
- Expose gel on a white phosphorimager screen at RT O/N.
- Scan exposed screen using a phosphorimager using manufacturer's protocol.
Representative Results
Figure 1 shows a representative result obtained with AFLIP. Signals in Lane 1 come from the 125I-ligand covalently linked to either the zebrafish betaglycan core protein (BG core, below the 150 kDa marker) or the BG core that has been processed to its proteoglycan form by attachment of glycosaminoglycans (GAG, smear ranging from 170 kDa to the top of the gel). This pattern of migration, a sharp core protein plus a smeared proteoglycan (due to heterogeneity in the length of the GAG chains), is characteristic of the TGFBR32. Since the DSS does not covalently link every single ligand-receptor complex formed, there is free 125I-ligand appearing at the migration front of the gel (125I-TGF-β2). This free ligand was bound to the receptor, and remained bound during the immunoprecipitation, but since it was not covalently attached, detached during the SDS-PAGE procedure. Nonetheless, the strength of its signal still correlates to the one given by the labeled receptor. This can be appreciated by comparing lanes 2 and 3. At 48 hpf the zebrafish embryos exhibit lower amounts of BG than 72 hpf embryos3, which can be seen by the faint signals at the GAG and BG core, that correspond to the intensity of the signals given by the free ligand appearing at the front of the gel (Lane 2). Given that antibodies against rat BG6 do not cross-react with the ZF BG, the antibodies when used in the AFLIP gave no signal, therefore, serving as a negative control (Lane 3). Similar negative results were obtained with the pre-immune serum of the anti-ZF BG antibody3.
Figure 2 shows how AFLIP can be used to gauge the levels of expression of a given receptor in embryos subjected to an experimental manipulation, in this case, the decrease of betaglycan due to morpholino injection3. Lane 1 shows the level of TGFBR3 in a 72 hpf wild type embryo, while Lane 2 shows the effect of administering 7 ng of a morpholino directed to the exon2-intron2 boundary of the ZF BG primary transcript. Note that the BG down regulation obtained with this morpholino is not reproduced by its mis-matched control (Lane 3). These results confirmed that the phenotype obtained with this morpholino was specific for the knockdown of the TGFBR3 as described before3.
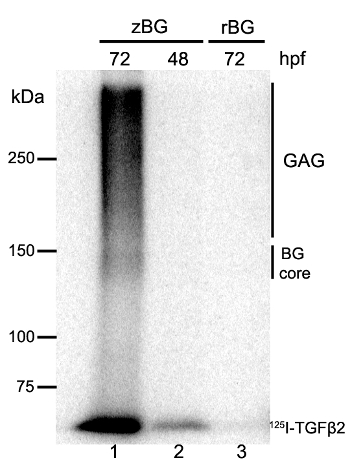
Figure 1. AFLIP Detection of BG in Zebrafish Embryos.Total protein extract from embryos at 72 hr post-fertilization (hpf) (Lanes 1 and 3) and 48 hpf (Lane 2) were subjected to 125I-TGF-β2 affinity labeling followed by IP with rabbit anti-zebrafish BG (Lanes 1 and 2, zBG) or rabbit anti-rat BG (Lane 3, rBG) as a control. In the autoradiography BG can be detected without GAG (BG core) or as a glycosaminoglycan-modified BG (GAG). Please click here to view a larger version of this figure.
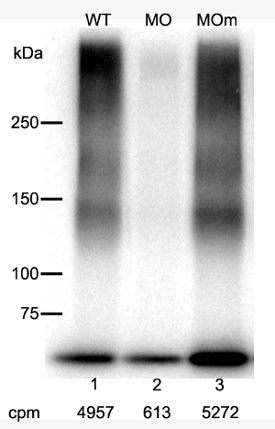
Figure 2. BG Morpholino Knockdown Detected by AFLIP. 125I-TGF-β2 affinity labeling of BG from untreated WT embryos (lane1), microinjected with specific BG morpholino (Lane 2) or a mismatch control (Lane 3). The counts per million (cpm) recovered after the immunoprecipitation and the cross-linking step are indicated. Please click here to view a larger version of this figure.
| Buffer | Composition |
| Buffer 1 | 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Triton X-100 |
| Deyolk buffer | 55 mM NaCl, 1.8 mM KCl, 1.25 mM NaHCO3 |
| Fish water | See Materials Table |
| Fixative solution | 50% CH3OH, 12% CH3COOH, 0.185% HCHO |
| IP wash buffer | 10 mM Na2HPO4 , 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, 0.1% Triton X-100, 0.02% SDS, pH 7.4 |
| Laemmli buffer | 2% SDS, 10% Glycerol, 100 mM DTT, 60 mM Tris (pH 6.8), 0.001% bromphenol blue. |
| Lysis buffer | 50 mM Tris-HCl pH7.4, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 0.5% Triton X-100, 0.1% SDS, 0.5% Sodium Deoxycholate |
| PBS | 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH7.4 |
| TNTE | 50 mM Tris-HCl pH7.4, 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100 |
| Washing buffer | 110 mM NaCl, 3.5 mM KCl, 2.7 mM CaCl2, 10 mM Tris-HCl pH 8.5 |
Table 1: Buffer Compositions
Discussion
The use of Western blots with a specific antibody against a protein of interest is a valuable tool to study its expression7 during embryogenesis. However, immunoblotting of highly-glycosylated proteins has not been very successful due to their inefficient transfer and weak binding to nitrocellulose or PVDF membranes8,9.
Proteoglycans are a good example of this shortcoming, because of their covalently attached glycosaminoglycan chains (GAG) that are negatively charged and do not bind well to either polystyrene surfaces or hydrophobic blotting membranes. Also, the size heterogeneity of the GAG chains "spreads" the protein over a sector of the gel, effectively decreasing its amount per area of the blot. In addition, if the protein of interest has low levels of expression, its detection by regular western blots is a difficult task. The type III transforming growth factor β receptor (TGFBR3) or betaglycan (BG), a membrane receptor with two GAG's attachment sites in mammals1 and one in zebrafish3, has all of these properties. Although, protocols to overcome these hurdles have been devised, like the detection by avidin-biotin complex (ABC) system10,11 or the immunobloting of proteoglycans on positively charged membranes12, they cannot resolve both problems in the same protocol. Taking advantage of the high affinity of BG for its natural ligand TGFβ2 and of the availability of a specific antibody, AFLIP, a technique that combines the advantages of affinity labeling and immunoprecipitation, the above discussed limitations of western blot detection may be overcome.
Affinity labeling of membrane-bound receptors with their radiolabeled ligands is a well used tool that has been instrumental for the identification and characterization of several important growth factors receptors in cultured cells13-16. While in conventional affinity labeling a monolayer of cells is subjected to covalent ligand labeling on the tissue culture dish and then lysed, in AFLIP the ligand-receptor complexes are first formed in embryo lysates, then purified by immunoprecipitation and finally, covalently cross-linked. Because of its use of radiolabeled ligands, AFLIP is very sensitive and in principle can be adapted to other growth factors or cytokines receptors for which a labeled ligand and a good antibody are available. This potential of AFLIP would help the detection of these low abundant but physiological relevant molecules.
Because of the inherent variability in the cross-linking step of affinity labeling, AFLIP cannot be used to quantitatively determine the levels of the measured receptors. However, if performed with adequate parallel controls, it can provide a quite accurate gauging of relative levels of their expression. As AFLIP use total protein extracts, it is not restricted to one specific cellular location of the receptor, it reveals its expression in the whole individual or organ, if applied to dissected adult specimens. Finally, if needed AFLIP could be linked to other biochemical protocols, like carbohydrate enzymatic digestions, to further characterize the receptor of interest.
Similar to conventional affinity labeling, a freshly 125I-labeled ligand is strongly recommended. Given the short half-life of 125I, the ligand must be used within the first 2 weeks after labeling with fresh isotope. Although most of the caveats of the protocol have been mentioned in the corresponding steps, one special mention shall be given to the careful removal of the yolk, which must be as complete and uniform as possible. Leaving uneven amounts of yolk would result in incorrect quantitation of bona fide embryo protein in the lysates.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors thank Gilberto Morales for fish care and maintenance, and Drs. Claudia Rivera and Hector Malagòn (IFC-UNAM Animal Facility) for their help in rabbit immunization. This work was supported by grants from CONACYT 131226 and PAPIIT-DGAPA-UNAM IN204916.
Materials
| Disuccinimidyl suberate (DSS) | ThermoFisher Scientific | 21555 | |
| Protein G Sephraose 4 Fast Flow | GE Healthcare Life Sciences | 17-0618-01 | |
| Gel Dryer Model 583 | BIO-RAD | 1651745 | |
| Typhoon 9400 | GE Healthcare Life Sciences | 63-0055-78 | |
| Cobra II Auto gamma counter | Packard | ||
| Exposure Cassette | Molecular Dynamics | 63-0035-44 | |
| NaCl | J.T. Baker | 3624 | |
| KCl | J.T. Baker | 3040 | |
| Na2HPO4 | J.T. Baker | 3828 | |
| K2HPO4 | J.T. Baker | 3246 | |
| CH4O | J.T. Baker | 9070 | |
| C2H4O2 | J.T. Baker | 9508 | |
| CH2O | J.T. Baker | 2106 | |
| SDS | Sigma-Aldrich | L4509 | |
| EDTA | Sigma-Aldrich | ED | |
| Triton X-100 | Sigma-Aldrich | T9284 | |
| CaCl2 | Sigma-Aldrich | C3306 | |
| NaHCO3 | Fisher Scientific | S233 | |
| PMSF | Sigma-Aldrich | P7626 | |
| Crystal Sea Marine Mix | Marine Enterprises International | http://www.meisalt.com/Crystal-Sea-Marinemix |
Riferimenti
- López-Casillas, F., et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-β receptor system. Cell. 67 (4), 785-795 (1991).
- Cheifetz, S., Andres, J. L., Massague, J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J. Biol. Chem. 263 (32), 16984-16991 (1988).
- Kamaid, A., et al. Betaglycan knock-down causes embryonic angiogenesis defects in zebrafish. Genesis. 53, 583-603 (2015).
- Bradford, M. M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 72, 248-254 (1976).
- Cheifetz, S., et al. Distinct transforming growth factor-b receptor subsets as determinants of cellular responsiveness to three TGF-b isoforms. J. Biol. Chem. 265, 20533-20538 (1990).
- López-Casillas, F., Wrana, J. L., Massagué, J. Betaglycan presents ligand to the TGFβ signaling receptor. Cell. 73 (7), 1435-1444 (1993).
- Towbin, H., Staehelin, T., Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. PNAS USA. 76 (9), 4350-4354 (1979).
- Maccari, F., Volpi, N. Direct and specific recognition of glycosaminoglycans by antibodies after their separation by agarose gel electrophoresis and blotting on cetylpyridinium chloride-treated nitrocellulose membranes. Electrophoresis. 24 (9), 1347-1352 (2003).
- Volpi, N., Maccari, F. Glycosaminoglycan blotting and detection after electrophoresis separation. Methods Mol Biol (Clifton, N.J). 1312, 119-127 (2015).
- Guesdon, J. L., Ternynck, T., Avrameas, S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 27 (8), 1131-1139 (1979).
- Bratthauer, G. L. The avidin-biotin complex (ABC) method and other avidin-biotin binding methods. Methods Mol Biol (Clifton, N.J). 588, 257-270 (2010).
- Heimer, R., Molinaro, L., Sampson, P. M. Detection by 125I-cationized cytochrome c of proteoglycans and glycosaminoglycans immobilized on unmodified and on positively charged Nylon 66. Anal. Biochem. 165 (2), 448-455 (1987).
- Das, M., et al. Specific radiolabeling of a cell surface receptor for epidermal growth factor. PNAS USA. 74 (7), 2790-2794 (1977).
- Massague, J., Guillette, B., Czech, M., Morgan, C., Bradshaw, R. Identification of a nerve growth factor receptor protein in sympathetic ganglia membranes by affinity labeling. J. Biol. Chem. 256 (18), 9419-9424 (1981).
- Massague, J., Pilch, P. F., Czech, M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. PNAS USA. 77 (12), 7137-7141 (1980).
- Hoosein, N. M., Gurd, R. S. Identification of Glucagon Receptors in Rat Brain. PNAS USA. 81, 4368-4372 (1984).

