Establishment of Proliferative Tetraploid Cells from Nontransformed Human Fibroblasts
Summary
Although proliferative polyploid cells are necessary to analyze chromosomal instability of polyploid cells, creating such cells from nontransformed human cells is not easy. The present report describes relatively simple procedures to establish proliferative tetraploid cells free of a diploid population from normal human fibroblasts.
Abstract
Polyploid (mostly tetraploid) cells are often observed in preneoplastic lesions of human tissues and their chromosomal instability has been considered to be responsible for carcinogenesis in such tissues. Although proliferative polyploid cells are requisite for analyzing chromosomal instability of polyploid cells, creating such cells from nontransformed human cells is rather challenging. Induction of tetraploidy by chemical agents usually results in a mixture of diploid and tetraploid populations, and most studies employed fluorescence-activated cell sorting or cloning by limiting dilution to separate tetraploid from diploid cells. However, these procedures are time-consuming and laborious. The present report describes a relatively simple protocol to induce proliferative tetraploid cells from normal human fibroblasts with minimum contamination by diploid cells. Briefly, the protocol is comprised of the following steps: arresting cells in mitosis by demecolcine (DC), collecting mitotic cells after shaking off, incubating collected cells with DC for an additional 3 days, and incubating cells in drug-free medium (They resume proliferation as tetraploid cells within several days). Depending on cell type, the collection of mitotic cells by shaking off might be omitted. This protocol provides a simple and feasible method to establish proliferative tetraploid cells from normal human fibroblasts. Tetraploid cells established by this method could be a useful model for studying chromosome instability and the oncogenic potential of polyploid human cells.
Introduction
Polyploidy has been observed not only in specialized tissues of mammalian species but also in a variety of pathological conditions, such as cancer and degenerative diseases. Polyploid (mostly tetraploid) cells are often observed in preneoplastic lesions of human tissues, such as Barrett's esophagus 1,2 or squamous intraepithelial lesions of the cervix 3,4, and have been considered to be the source of malignant aneuploid cells in those tissues 5,6. Although it is suggested that conversion of tetraploid to aneuploid cells could be a crucial event in the early stages of tumorigenesis, the mechanisms involved in this process are not fully understood. This is partly because no in vitro model has been available where nontransformed polyploid human cells can propagate.
Some researchers have induced tetraploidy in nontransformed human epithelial cells through generation of binucleated cells by inhibiting cytokinesis7-9. In this method, however, unnecessary diploid cells must be eliminated by fluorescence-activated cell sorting (FACS) 7,8 or cloning by limiting dilution 9. Because these procedures are laborious and not easy to perform, simpler methods to establish nontransformed tetraploid cells are desired for research in this field.
In the present report, we describe a protocol to establish proliferative tetraploid cells from normal human fibroblasts or telomerase-immortalized human fibroblasts by relatively simple procedures. The procedures use spindle poison demecolcine (DC) to arrest diploid cells in mitosis, and mitotic cells collected by shaking off are further treated with DC. Diploid mitotic cells treated with DC for prolonged time convert to tetraploid G1 cells, and these cells proliferate as tetraploid cells after growth arrest for several days following drug removal. This protocol provides an efficient method for creating a useful model to study the relationship between chromosome instability and the oncogenic potential of polyploid human cells.
Protocol
1. Cell Culture
- Obtain the cells to induce tetraploidy. To date it has been confirmed that this technique can be applied to the human fibroblast cell lines TIG-1, BJ, IMR-90 and telomerase-immortalized TIG-1 (TIG-hT).
- Grow cells in minimum essential medium with α modification or any other cell culture medium suited for the cell type to be studied supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) by incubating in a 5% (v/v) CO2 atmosphere at 37 °C. Passage cells every 3 or 4 days not to exceed subconfluent density.
NOTE: Population doubling level (PDL) should be calculated each time at passaging to evaluate cell growth and cell age. PDL can be calculated from the cell number seeded into a dish (N1) and the cell number harvested at the next passaging (N2) using the following formula (X is the initial PDL). PDL = X + (log N1 – log N2) / log 2
2. Induction and Establishment of Tetraploid Cells from Diploid Fibroblasts
- Induce tetraploidy without shake-off.
NOTE: Some cell strains (e.g. TIG-1) can become almost completely tetraploid by continuous treatment with demecolcine (DC) as follows.- Passage cells into one or two 100 mm dishes on the day before treatment to induce tetraploidy. Usually, prepare 5 x 105 to 1 x 106 cells per dish for induction of tetraploid cells.
- Treat exponentially growing cells with medium containing 0.1 µg/mL DC for 4 days.
- Replace DC-containing medium with drug-free medium and incubate cells in a 5% (v/v) CO2 atmosphere at 37 °C. Cells usually resume proliferation within 1 week.
- Induce tetraploidy with shake-off.
NOTE: Most cells become a mixture of diploid and tetraploid populations as a result of the simple treatment with DC described above (2.1). Therefore, some modifications of the procedure to eliminate a diploid population are necessary as follows (Figure 1).- Passage cells into several T75 flasks the day before treatment to induce tetraploidy. Usually, prepare at least 5 x 106 cells (1 x 106 cells per flask) for induction of tetraploid cells.
- Treat exponentially growing cells with medium containing 0.1 µg/mL DC for 16 – 18 h to arrest cells in mitosis. The time required to arrest cells in mitosis may depend on target cells and should be determined by preliminary experiments to obtain as many mitotic cells as possible. For example, slower growing cells should be treated with DC for longer time than faster growing cells.
NOTE: If cells are treated for too long in this step, they might undergo mitotic slippage and adhere to the dish surface, making it impossible to collect mitotic cells by shake-off in the next step. - Collect DC-arrested mitotic cells by the shake-off method, in which loosely adhered mitotic cells are collected by gentle shaking of flasks and washing with medium followed by centrifugation (300 x g, 5 min). Count cell number by an appropriate method in this step.
- Reseed collected mitotic cells into 60 mm culture dishes and treat cells with 0.1 µg/mL DC for an additional 3 days. Seed more than 1 x 106 mitotic cells per dish, because less than 10% of cells can survive this treatment.
- Replace DC-containing medium with drug-free medium and wait for cells to resume proliferation, which usually occurs within 1 week.
- Passage cells similarly to original diploid cells.
3. Examination of DNA Ploidy
NOTE: This is a well-established procedure and a technical manual should be referred to for detailed procedures and technical tips.
- Harvest cells (5 x 105 – 1 x 106) by incubating cells with a solution containing 0.05% trypsin and 0.02% EDTA for 10 min at 37 °C, and neutralize trypsin by medium containing 10% FBS.
- Centrifuge cells in medium and wash them by resuspending in 5 mL of phosphate-buffered saline (PBS) followed by centrifugation (300 x g, 5 min).
- Prepare cells for DNA analysis by one of the following two methods
- A method for analyzing unfixed cells10
NOTE: This method can be performed using a commercially available kit for cell cycle analysis. Samples prepared with this method should be analyzed immediately, because cells are not fixed in the procedure and stained nuclei will become damaged over time.- Wash cells with 40 mM citrate buffer containing 250 mM sucrose. Subsequently treat cells with 250 µL of 0.1% (v/v) Nonidet P40 and 30 µg/mL trypsin in 200 µL of 40 mM citrate buffer containing 250 mM sucrose for 10 min at room temperature.
- Add 200 µL of 0.5 µg/mL trypsin inhibitor and 0.1 µg/mL RNase A in 40 mM citrate buffer containing 250 mM sucrose, and incubate cells for 10 min at room temperature.
- Add 200 µL of 0.4 mg/mL propidium iodide (PI) in 40mM citrate buffer containing 250 mM sucrose, and incubate cells for 10 min at room temperature to stain cell nuclei.
- A method for analyzing fixed cells
- Fix cells with 80% ethanol by suspending cells in 1 mL of PBS, adding 4 mL of 100% EtOH dropwise while mixing the cell suspension and leaving it at room temperature for 30 min. Cells in this fixative can be stored at -20 °C for several weeks.
- Wash cells by resuspending them in 5 mL of PBS followed by centrifugation (300 x g, 5 min). Treat cells with 0.5 mg/mL RNase A and stain them with 50 µg/mL propidium iodide (PI) (475 µL of 0.5 mg/mL RNase A and 25 µL of 1 mg/mL PI) for 30 min at room temperature.
- A method for analyzing unfixed cells10
- Analyze DNA content of the cells using a flow cytometer with a 488 nm laser and red channel emission filter (long pass > 610 nm). Analyze a reference sample prepared from genuine diploid cells at the same time to assess ploidy of the target cells.
- Alternatively, add fluorescence standard beads to assess the fluorescence intensity ratio of diploid cells vs. the beads. Use the 'pulse height vs. pulse width' option of the flow cytometer to discriminate between single tetraploid cells and clumps of diploid cells11.
4. Examination of Chromosome Counts and Karyotype Analysis
NOTE: These are all well-established procedures and a technical manual or the manufacturer's protocols should be referred to for detailed procedures and technical tips.
- Treat exponentially growing cells with 0.1 µg/mL DC for 4 h to arrest cells in metaphase.
- Prepare chromosome slides.
- Harvest cells (at least 5 x 105 cells) by trypsinization and suspend in medium containing FBS.
- Centrifuge cells in medium (300 x g, 5 min) and treat with hypotonic solution (5 mL of 0.075 M KCl) at 37 °C for 10 min.
- Fix and suspend cells in Carnoy's fixative (methanol:acetic acid = 3:1).
- Spread cells onto a glass slide by dropping a small volume (25 – 35 µL) of cell suspension. Additional steps such as exposure to hot steam might be necessary to improve chromosome spreading.
- Stain cells for chromosome counts or karyotype analysis or multicolor fluorescence in situ hybridization (mFISH).
- Chromosome counts.
- Stain cells with 5% Giemsa solution (or 5 µg/mL PI containing 0.5 mg/mL RNase A for fluorescence microscopy) for 15 – 20 min.
- Digitally photograph at least 50 metaphase cells.
- Count chromosome number per cell using an image analysis software after manual separation of touching and overlapping chromosomes using a photo editing software. Although chromosome number can be scored manually, computerized scoring is recommended.
- Karyotype analysis
NOTE: This analysis should be performed by a trained technician or a professional company.- Stain cells according to a standard G-banding technique12.
- Analyze chromosomes to make standard karyograms12.
- mFISH
NOTE: This step is optional and should be performed if necessary.- Stain cells using multicolor FISH probes for human chromosomes according to the manufacturer's protocol13.
- Analyze chromosomes using a fluorescence microscope with suitable filters and a software for mFISH analysis13.
- Chromosome counts.
Representative Results
In our experience, TIG-1 cells can be made almost completely tetraploid by simple continuous treatment with 0.1 µg/mL DC for 4 days (Figure 2A). In contrast, other fibroblast strains, such as BJ or IMR-90, and TIG-hT cells, became a mixture of diploid and tetraploid populations following the same treatment, and isolation of mitotic cells by the shake-off method is necessary during the course of DC treatment (usually 16 – 18 h after the start of treatment) (Figures 2B, C). After an additional treatment with DC for 3 days, cells underwent growth arrest and exhibited large, flattened morphology for several days. Within one week in general, small proliferating cells appeared among the large flattened cells (Figure 3). Cells became almost completely tetraploid in 2 – 3 weeks after DC treatment. The modal chromosome number in established tetraploid cells was 92 in most cases, except that one of the tetraploid cell lines established from TIG-hT cells had 91 chromosomes in the majority of cells (Figure 4). Established tetraploid cells grew at almost the same rate as diploid cells (Figure 5). Tetraploid cell lines established from non-immortalized cells showed an approximately equal replicative lifespan as the original diploid cells, while those from immortalized cells seemed to be also immortal (Figure 5). Tetraploid cells established from non-immortalized TIG-1 cells showed a normal karyotype except for having a tetraploid chromosome number (Figure 6 top panel), whereas those from immortalized TIG-1 (TIG-hT) cells showed rather complicated clonal aberrations (Figure 6 middle and bottom panels). The frequency of clonal aberrations in the latter cells (TIG-hT-4n) increased from 2.5 aberrations per cell at 255 population doubling levels (PDLs) to 5.2 aberrations per cell at 450 PDL with repeated subculture.
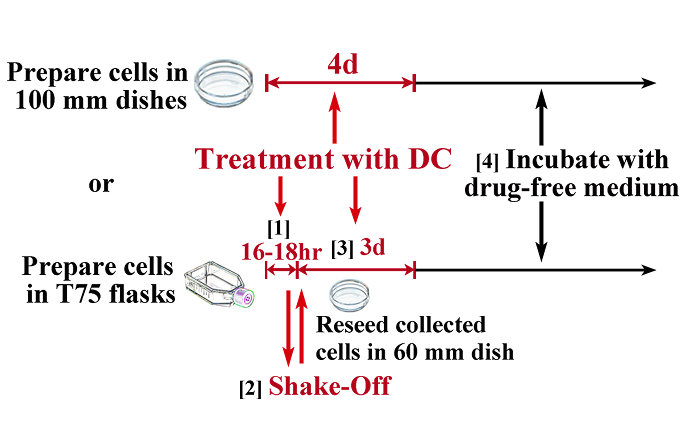
Figure 1: Schematic Illustration of the Protocol for Induction of Tetraploidy. To obtain tetraploid cells with minimum contamination of diploid cells, a combination of mitotic shake-off and DC treatment is necessary for most cell strains (lower illustration), while some cell strains (e.g. TIG-1) can become almost completely tetraploid by continuous treatment with demecolcine (upper illustration). 1) Treat cells in T75 flasks (at least 5 x 106) with 0.1 µg/mL DC for 16 – 18 h (this time should be determined by preliminary experiments) to arrest cells in mitosis. 2) Collect mitotic cells by the shake-off method and reseed into 60 mm culture dishes. 3) Treat cells with 0.1 µg/mL DC for an additional 3 days. 4) Incubate cells in drug-free medium (usually cells resume proliferation within 1 week). Scale bars represent 100 µm. Please click here to view a larger version of this figure.
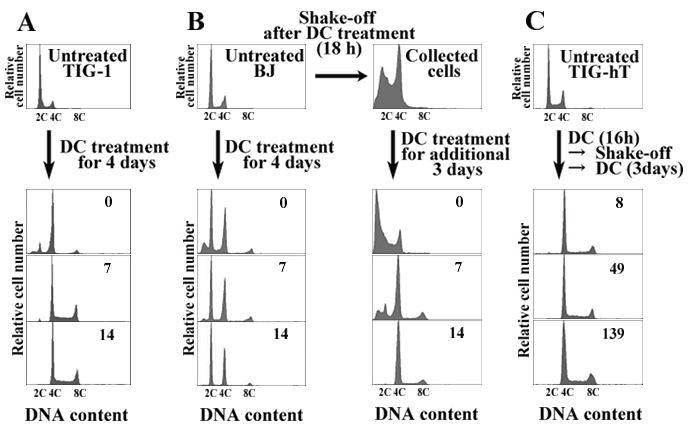
Figure 2: DNA Histograms of Fibroblasts Before and After Treatments for Induction of Tetraploidy. (A) TIG-1 cells. This cell strain becomes tetraploid by simple continuous treatment with 0.1 µg/mL DC for 4 days. (B) BJ cells. Because this cell strain becomes a mixture of diploid and tetraploid populations following simple treatment with DC (left panels), isolation of mitotic cells by shaking off is necessary during DC treatment to establish tetraploid cells (right panels). (C) TIG-hT (telomerase-immortalized TIG-1) cells. These cells also need to be prepared by a combination of DC treatment and shake-off for establishment of tetraploid cells. Numerals in the histograms represent the time (days) after drug removal. The abscissa represents DNA content (C, complement). Please click here to view a larger version of this figure.
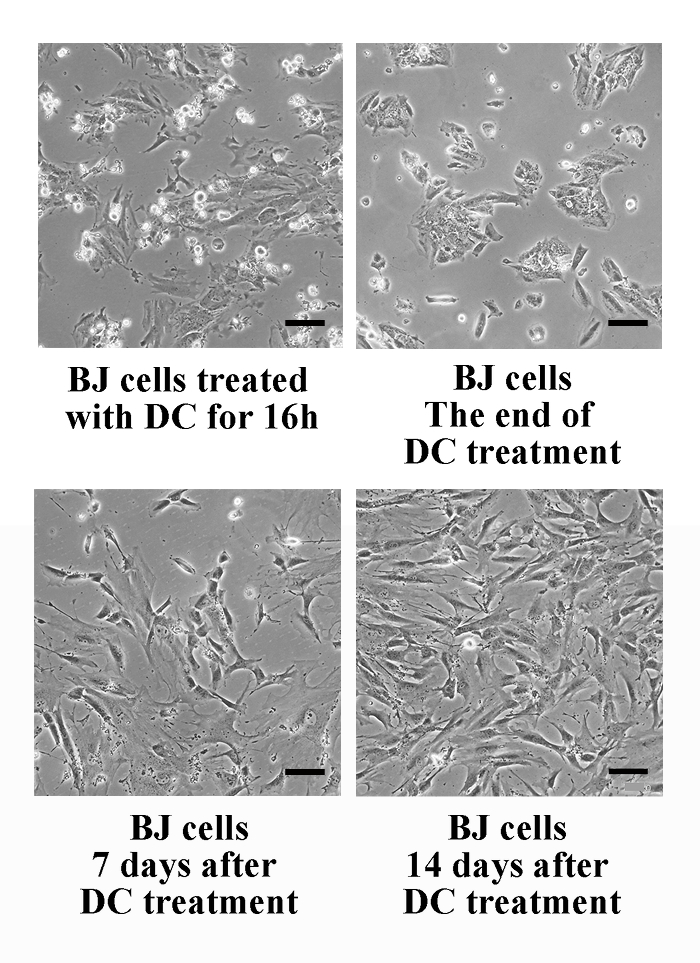
Figure 3: Representative Photomicrographs of BJ Cells Treated with DC. (A) BJ cells treated with 0.1 μg/mL DC for 16 h. Many cells arrested in mitosis can be seen. (B) The end of DC treatment. Almost all cells are growth-arrested. (C) 7 days after DC treatment. Small proliferating cells among large flattened cells can be seen. (D) 14 days after DC treatment. Cells are actively growing. Please click here to view a larger version of this figure.
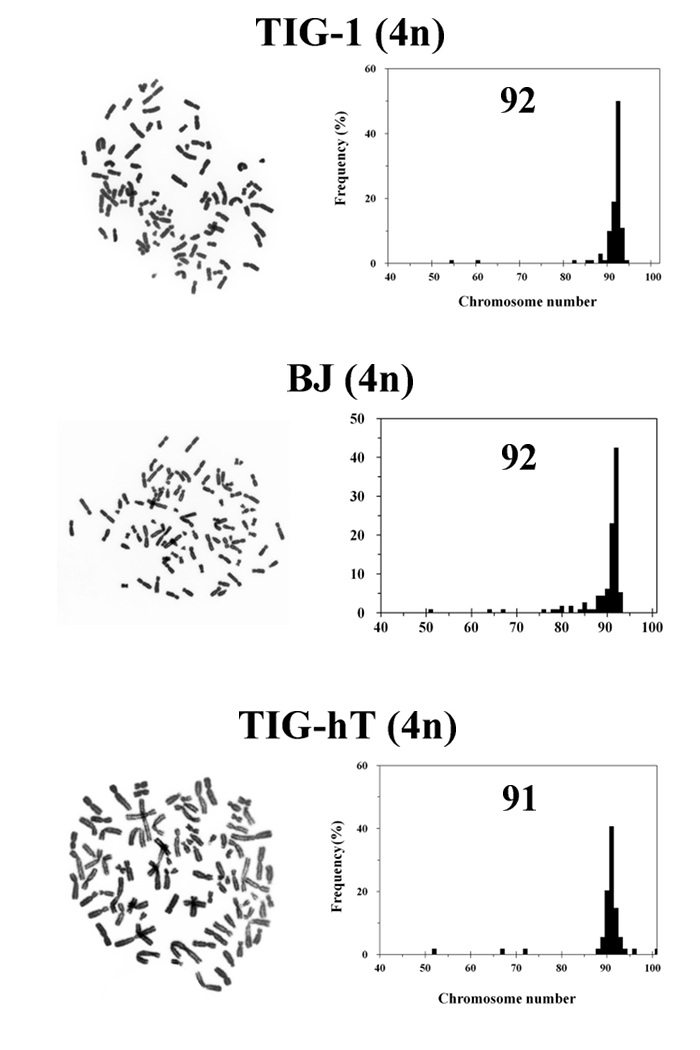
Figure 4: Representative Photomicrographs of Chromosomes and Histograms of Chromosome Number for Tetraploid Cells Established From Each Cell Strain. Top, middle and bottom panels represent tetraploid cells established from TIG-1 (2 weeks after DC treatment), BJ (2 weeks after DC treatment) and TIG-hT (7 weeks after DC treatment) cells respectively. Chromosomes were scored in at least 50 metaphases. Numerals in histograms are modal chromosome numbers. Please click here to view a larger version of this figure.
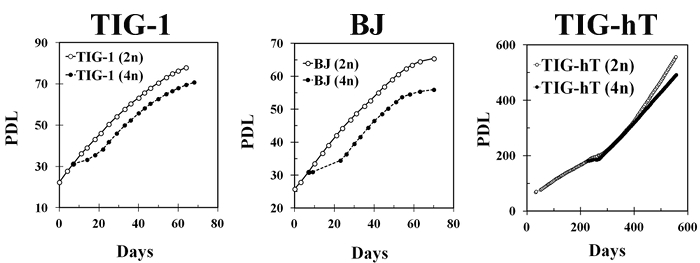
Figure 5: Representative Growth Profiles of Human fibroblasts (Diploid Cells) and Tetraploid Cells Established from Each Cell Strain. Left, center and right panels show growth profiles of original TIG-1, BJ and TIG-hT cells (open markers) and those of tetraploid cells established from each cell strain (closed markers). Cells were passaged twice a week and PDLs were calculated from cell numbers. Please click here to view a larger version of this figure.
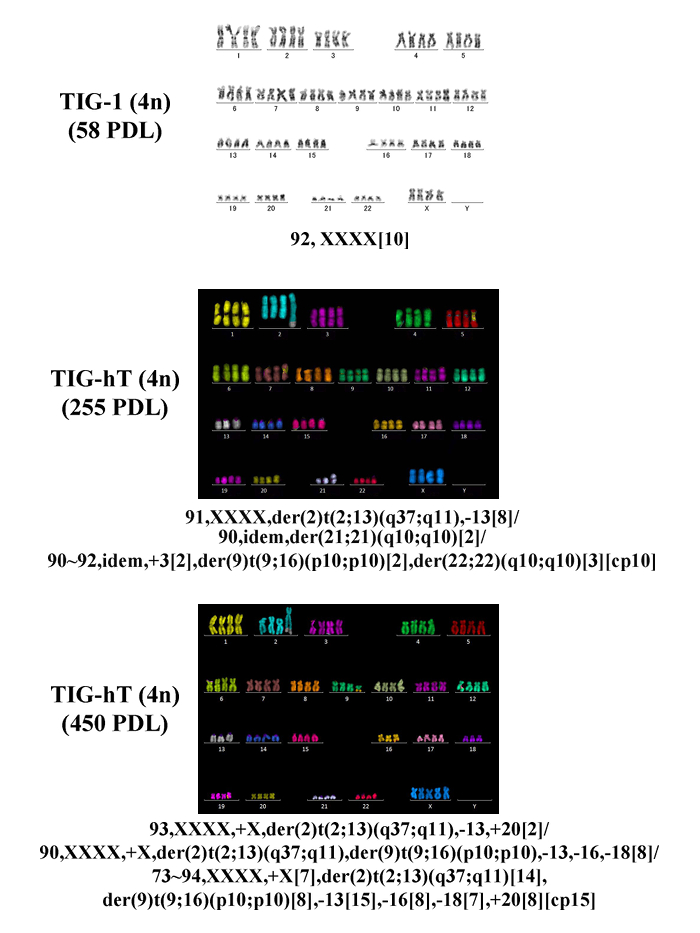
Figure 6: Representative Karyograms by G-banding and mFISH. Top panel shows a karyogram by G-banding of tetraploid cells established from TIG-1 (2 weeks after DC treatment). Middle and bottom panels show karyograms by mFISH for tetraploid cells established from TIG-hT cells (15 and 41 weeks after DC treatment). Karyotypes were based on 10 cells (TIG-1) or 20 cells (TIG-hT). Please click here to view a larger version of this figure.
Discussion
A major problem in induction of tetraploidy from diploid cells by chemical agents, either by cytokinesis inhibitors or by spindle inhibitors, is that cells often become a mixture of diploid and tetraploid populations, and tetraploid cells must be separated from diploid cells. Most common approaches for isolation of a tetraploid population free of diploid cells use FACS or cloning by limiting dilution. However, these procedures are laborious and not easy to perform. In this report, we present a new protocol to establish proliferative tetraploid cells free of a diploid population from normal human fibroblasts. The protocol uses spindle poison DC for arresting diploid cells in mitosis, and arrested mitotic cells are separated by shaking-off from diploid interphase cells, which adhered to the dish surface. Collected cells are treated with DC for an additional 3 days, and diploid mitotic cells convert to tetraploid G1 cells by this prolonged DC treatment presumably by checkpoint adaptation (also called mitotic slippage). These cells restart cell growth as tetraploid cells after growth arrest for several days of drug removal.
An advantage of this method is that the procedures are relatively simple and feasible compared with those employing FACS or cloning to isolate tetraploid cells. Limitations of the method are the following. Applicability to cell types other than fibroblasts, such as epithelial cells, is unknown at present. This should be confirmed in future studies. A small diploid population might remain in some cases. However, these cells tend to diminish with serial passaging. If a significant diploid population persists after DC treatment, a higher concentration of DC (1.2 – 1.5 µg/mL) or longer treatment with DC after the shake-off (4 days instead of 3 days) might improve the result.
The most critical steps in this protocol are the following. Firstly, treatment time with DC for arresting cells in mitosis before shake-off is critical. Although this treatment time should be long enough to collect as many mitotic cells as possible, treating for too long might cause mitotic slippage and adhesion to the dish surface, making it impossible to collect the mitotic cells by shaking off. Because an appropriate time for the maximum recovery of mitotic cells may depend on the target cells, it should be determined by preliminary experiments. Secondly, the shaking off step to separate mitotic cells from interphase cells is also critical. In this step, diploid mitotic cells, which are loosely adhered to the dish surface, are collected by gentle shaking of the flasks followed by centrifugation. This mitotic shake-off separates mitotic cells arrested by DC from adhered interphase cells that might be the origin of contamination by the diploid population later. Shaking violently may cause bubbling of the medium, which might damage cells, and also detachment of interphase cells, resulting in contamination of diploid cells. Another factor that may affect the success of this method is the culture medium. Because robust growth stimulation seems to be necessary for the recovery of proliferation after DC treatment, nutrient-rich medium such as MEM-α should be used to support proliferation of tetraploid cells. Increasing FBS concentration to 20% at the final step of induction of tetraploid cells (2.2.5) might also increase the success of this method.
Using this protocol, we have to date produced tetraploid cells from the human fibroblast strains TIG-1, BJ, IMR-90 and telomerase-immortalized TIG-1 up to now 14-16. Among these cell strains, TIG-1 is rather unusual because this cell strain becomes almost completely tetraploid when treated continuously with DC, without shake-off, for 4 days. The reason why only TIG-1 cells become completely tetraploid as a result of simple treatment with DC is unknown at present. Possible explanations might be that a significant proportion of cells of other types arrests at diploid G1 phase when treated with DC and resumes proliferation after treatment, whereas (1) TIG-1 cells do not arrest at G1 with the same treatment, or (2) a population of TIG-1 cells irreversibly arrests at G1 under the same treatment and does not resume proliferation thereafter. In any case, different sensitivities of the G1 checkpoint to DC might be responsible for the different behaviors of the cells after continuous treatment with DC.
When investigating the etiological significance of tetraploidy in tumorigenesis, many researchers have great concerns about the status of the p53 gene in tetraploid cells, because it has been proposed that tetraploidy induces p53-dependent growth arrest of cells, which is termed the "tetraploidy checkpoint" 17-20. If this hypothesis is correct, p53 signaling in proliferating tetraploid cells should be inactivated. However, tetraploid cells established by our protocol seem to have functional p53 despite growing at almost the same rate as diploid cells 15,16. The reason for this discrepancy is not known at present, and significance of p53 inactivation in the proliferation of tetraploid cells should be examined precisely in future studies. In fact, the presence of a tetraploidy checkpoint that functions through p53 remains controversial 21-23.
Although nontransformed polyploid cells that can propagate in vitro are indispensable for detailed analysis of chromosomal instability and the oncogenic potential of polyploid cells, such cells have so far rarely been available. Our protocol provides a simple and feasible method to establish proliferative tetraploid cells from normal human fibroblasts, which could be a useful model for studying the mechanisms leading to transformation of polyploid human cells.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Mrs. Matsumoto for the technical assistance.
Materials
| MEM-α | Sigma-Aldrich | M8042-500ML | |
| Trypsin-EDTA | Sigma-Aldrich | T4174 | |
| FBS | Sigma-Aldrich | 172012-500ML | |
| Demecolcine solution (10 μg/mL in HBSS) | Sigma-Aldrich | D1925-10ML | |
| BD CycleTES Plus DNA Reagent Kits | BD Biosciences | #340242 | For examination of DNA ploidy by flow cytometry |
| Human chromosome multicolor FISH probe 24XCyte | MetaSystems | #D-0125-060-DI | Specialized filter set and software for mFISH analysis are necessary |
| Isis imaging system with mFISH software | MetaSystems | Specialized probe kit is necessary |
Riferimenti
- Rabinovitch, P., et al. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am. J. Gastroenterol. 96 (11), 3071-3083 (2001).
- Galipeau, P., et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 4 (2), e67 (2007).
- Olaharski, A., et al. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 27, 337-343 (2006).
- Liu, Y., et al. p53-independent abrogation of a postmitotic checkpoint contributes to human papillomavirus E6-induced polyploidy. Cancer Res. 67, 2603-2610 (2007).
- Davoli, T., de Lange, T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 27, 585-610 (2011).
- Fox, D., Duronio, R. Endoreplication and polyploidy: insights into development and disease. Development. 140, 3-12 (2013).
- Fujiwara, T., et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 437, 1043-1047 (2005).
- Ganem, N., et al. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 158 (4), 833-848 (2014).
- Kuznetsova, A., et al. Chromosomal instability, tolerance of mitotic errors and multidrug resistance are promoted by tetraploidization in human cells. Cell cycle. 14 (17), 2810-2820 (2015).
- Vindeløv, L., Christensen, I. Detergent and proteolytic enzyme-based techniques for nuclear isolation and DNA content analysis. Methods Cell Biol. 41, 219-229 (1994).
- Darzynkiewicz, Z., Juan, G. DNA content measurement for DNA ploidy and cell cycle analysis. Curr Protoc Cytom. , Chapter 7: Unit 7.5 (2001).
- Knutsen, T., Bixenman, H., Lawce, H., Martin, P. Chromosome analysis guidelines preliminary report. Cancer Genet Cytogenet. 52 (1), 11-17 (1991).
- Liehr, T., et al. Multicolor FISH probe sets and their applications. Histol. Histopathol. 19 (1), 229-237 (2004).
- Ohshima, S., Seyama, A. Formation of bipolar spindles with two centrosomes in tetraploid cells established from normal human fibroblasts. Hum. Cell. 25 (3), 78-85 (2012).
- Ohshima, S., Seyama, A. Establishment of proliferative tetraploid cells from normal human fibroblasts. Front. Oncol. 3, 198 (2013).
- Ohshima, S., Seyama, A. Establishment of proliferative tetraploid cells from telomerase-immortalized normal human fibroblasts. Genes, Chromosome Cancer. 55 (6), 522-530 (2016).
- Di Leonardo, A., et al. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res. 57, 1013-1019 (1997).
- Andreassen, P., Lohez, O., Lacroix, F., Margolis, R. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell. 12, 1315-1328 (2001).
- Vogel, C., et al. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 23, 6845-6853 (2004).
- Aylon, Y., Oren, M. p53: Guardian of ploidy. Mol. Oncol. 5 (4), 315-323 (2011).
- Uetake, Y., Sluder, G. Cell cycle progression after cleavage failure : mammalian somatic cells do not possess a "tetraploidy checkpoint". J. Cell Biol. 165, 609-615 (2004).
- Ganem, N., Pellman, D. Limiting the proliferation of polyploid cells. Cell. 131, 437-440 (2007).
- Ho, C., Hau, P., Marxer, M., Poon, R. The requirement of p53 for maintaining chromosomal stability during tetraploidization. Oncotarget. 1 (7), 583-595 (2010).

