Determining the Optimal Inhibitory Frequency for Cancerous Cells Using Tumor Treating Fields (TTFields)
Summary
Tumor Treating Fields (TTFields) are an effective anti-tumor treatment modality delivered via the continuous, noninvasive application of low-intensity, intermediate-frequency, alternating electric fields. TTFields application to cell lines using a TTFields in vitro application system allows for the determination of the optimal frequency that leads to the highest reduction in cell counts.
Abstract
Tumor Treating Fields (TTFields) are an effective treatment modality delivered via the continuous, noninvasive application of low-intensity (1-3 V/cm), alternating electric fields in the frequency range of several hundred kHz. The study of TTFields in tissue culture is carried out using the TTFields in vitro application system, which allows for the application of electric fields of varying frequencies and intensities to ceramic Petri dishes with a high dielectric constant (Ɛ > 5,000). Cancerous cell lines plated on coverslips at the bottom of the ceramic Petri dishes are subjected to TTFields delivered in two orthogonal directions at various frequencies to facilitate treatment outcome tests, such as cell counts and clonogenic assays. The results presented in this report demonstrate that the optimal frequency of the TTFields with respect to both cell counts and clonogenic assays is 200 kHz for both ovarian and glioma cells.
Introduction
Tumor Treating Fields (TTFields) are an anti-mitotic modality for the treatment of glioblastoma multiforme and potentially other cancer types. The fields are delivered via the continuous application of low-intensity (1-3 V/cm), intermediate-frequency (100-500 kHz), alternating electric fields to the region of the tumor1,2. TTFields application in vitro and in vivo was shown to inhibit both the growth of various cancerous cell lines and the progression of the tumors in several animal tumor models1,2,3,4,5,6,7. Pilot clinical trials and larger randomized studies in patients with solid tumors, including glioblastoma and non-small cell lung cancer, have demonstrated the safety and efficacy of continuous TTFields application8,9,10. The efficacy of the TTFields was found to be: (1) frequency-dependent, with specific optimal frequencies leading to the highest reduction in the cell counts of cell lines from different origins1,2,4,5,6,7; (2) electric field intensity-dependent, with a minimal threshold for activity at around 1 V/cm and more potent higher intensities1,2,7,11; (3) enhanced when the treatment duration was longer5; and (4) higher when 2 directional TTFields were applied perpendicularly to each other, as compared to electric fields applied from a single direction1. Based on the above findings, TTFields can be applied to patients for long durations using 2 sets of transducer arrays localized on the patients' skin to maximize the electric field intensities in the tumor bed12,13.
Studying the effects of TTFields on cancerous cells in vitro currently provides the only way to determine the optimal frequency to apply to a specific tumor type. Testing for the optimal frequency requires a device that allows for the application of different frequencies in the range of 100-500 kHz and at intensities of up to 3 V/cm root mean square (RMS) to the cell culture. As TTFields application produces heat, the application system requires the ability to dissipate excessive heat while maintaining tight control over the temperature.
Several devices were developed throughout the years to allow for TTFields application to cell cultures1,2,5,14,15,16. In all of these devices, the electrodes used were insulated in order to avoid the caveats involved with the use of conductive electrodes, such as electron exchange at the electrode surface and the release of toxic metal ions into the medium1. The main difference between the various TTFields application systems tested is the type of electrode insulation used, with either electrodes made of metal wires insulated with a thin film of insulator2,14,15,16 or with a high dielectric-constant material (e.g., lead magnesium niobate-lead titanate (PMN-PT))6. While the insulated-wire electrodes offer a relatively simple and cost-effective solution for TTFields application, they are often limited by the high voltage required to achieve effective electric field intensities above the 1 V/cm threshold and by the surface available for cell plating, as the distance between the electrodes is relatively small. Systems based on electrodes insulated using a high dielectric-constant material require special design and manufacturing capabilities, yet they do not require high voltage and can offer a larger area for cell growth between the electrodes.
The TTFields in vitro application system used in this work belongs to the latter class of systems, with the core unit being a Petri dish (TTFields dish, see Figure 1) composed of high dielectric-constant ceramic (i.e., PMN-PT). Two pairs of electrodes are printed perpendicularly on the outer walls of a TTFields dish to allow for the application of electric fields from 2 directions. The electrodes are connected to a sinusoidal waveform generator and an amplifier, which allow for TTFields application in the frequency range of 50-500 kHz. In order to dissipate the excessive heat, the TTFields dishes are kept inside a refrigerated incubator, with the medium temperature-control performed using constant monitoring of the dish temperature and adjustments to the voltage applied by the system. In practice, setting the incubator to a lower temperature would lead to higher electric field intensities, as the system increases the voltage until the target temperature within the dish is achieved. The difference between the temperature within the dish and the incubator temperature may lead to some evaporation, depending on the temperature gradients; hence, the culture medium needs to be replaced every 24 h to maintain adequate growth conditions.
The protocol below describes the experimental procedure to optimize the application of TTFields frequencies to cancerous cells so that a maximum reduction in cell count and a reduction in the potential of the surviving cells to form colonies are achieved.
Protocol
1. TTFields In Vitro Application System — Base Plate and Dish Maintenance
- Rinse dishes and dish covers under running tap water. Then rinse with deionized water and air-dry face down.
- If a chemotherapeutic agent/drug has been added to dishes in a prior experiment, fill each dish with a 5% light detergent solution and leave overnight.
- Rinse the dishes thoroughly under running tap water to avoid any traces of detergent inside the dish. Rinse the dishes and dish covers with deionized water and air-dry face down.
- Insert the clean and dry dishes with their covers into sterilization bags. Seal and place the bags in an autoclave, with the dishes face down. Autoclave for 30 min at no higher than 121 °C.
- Set the autoclave on a dry program, partly open the autoclave door, and dry the dishes for 30 min.
- Wipe the base plate with a cloth lightly moistened with 70% ethanol.
2. Experiment Setup
NOTE: All equipment and materials used in this protocol are described in the Materials List. All steps below should be performed inside a laminar flow cabinet while sterile conditions are maintained.
- Prepare clean and sterile TTFields dishes and covers, as described in section 1. Wipe the base plate with a cloth lightly moistened with 70% ethanol.
- Install the TTFields dishes with the covers onto the base plate by pressing down gently on the dish and rotating clockwise by about 5 mm until the rim of the dish locks onto the three pins on the base plate. Place a sterile 22-mm coverslip (treated plastic or glass) on the bottom of each dish.
- Prepare the cell suspension in complete growth medium (Dulbecco's modified Eagle's medium for U-87 MG and F98; RPMI for A2780 and OVCAR-3; supplemented as described in Materials List).
NOTE: The cell concentrations depend on the cell types and experiment duration, 80%-90% confluent cell growth should not be exceeded by the end of the experiment.
CAUTION: Human cell lines may represent a biological hazard and should be handled with the appropriate safety measures. - Place 200 µL of cell suspension (usually 5,000-20,000 cells in 200 µL for a 72-h experiment, depending on the doubling time) as a drop in the center of each coverslip and cover with the dish lids.
- Incubate at 37 °C in a CO2 incubator until the cells adhere; an overnight incubation is possible at this stage.
- Aspirate the fluids from the drop using a 200- or 1,000-µL pipette.
- Gently pipette 2 mL of complete growth medium to fill each dish. Use a sterile tip to pipette and softly tap on the coverslip edges to release the air bubbles occasionally caught under the slide.
- Cover the dishes with their lids and place them inside a CO2 incubator at 37 °C until the start of the TTFields treatment.
3. TTFields Application
- Transfer the base plates with the attached TTFields dishes to a refrigerated CO2 incubator.
NOTE: In vitro TTFields application will generate heat; therefore, a refrigerated incubator is required to compensate for the warming of the dishes. Higher TTFields intensities will produce more heat and will require lower incubator ambient temperatures (see Table 1). Clinically relevant TTFields intensities are achieved when the incubator temperature for TTFields application is in the range of 18-30 °C. - Connect the flat cable female connector to the base plate. Switch the TTFields generator on.
- Start the TTFields in vitro application system software and select the experiment settings.
- Define a new experiment. Type the name of the experiment and the experiment owner in the software user interface on the computer screen. Adjust the frequency and target temperature for each dish or base plate.
- Start the TTFields application using the software.
- Verify that all dishes are connected properly and appear light blue on the monitor. If a dish is circled with a red frame (meaning that it is not properly connected), press it down gently and rotate slowly back and forth until the contacts are restored and the dish appears light blue.
- Leave the TTFields in vitro application system running for up to 24 h.
- If the experiment reaches its endpoint, stop the experiment by clicking on the END EXPERIMENT button in the software and proceed to step 5. If not, click on PAUSE experiment.
- Disconnect the flat cable connector from the base plate. Remove the base plate with the dishes from the incubator into a laminar flow cabinet.
- Replace the medium in all dishes every 24 h and return the base plate to the refrigerated incubator. Connect the base plate to the generator using the flat cable. Continue the experiment by clicking on the CONTINUE button.
4. Control Samples
Note: Grow control cells in similar conditions to TTFields-treated cells, excluding TTFields application.
- Plate control samples with the same suspension and on a similar surface used for plating TTFields-treated samples.
- Place control dishes in a CO2 incubator at 37 °C.
- Replace the medium in the dishes every 24 h to match the TTFields-treated dish medium conditions.
5. Experiment End
- After clicking on END EXPERIMENT, wait for the software to retrieve all records from the system and to save the records to the computer.
- Use the REPORTS button to review the temperature, current, and resistance log files of each base plate to verify that all dishes were treated according to the experimental plan.
NOTE: All reports and logs are saved to the computer after the end of the experiment and can be reviewed at any time. - Switch off the TTFields generator.
- Disconnect the flat cable from the base plate and remove from the incubator.
- To remove a ceramic dish, press down and turn the dish counterclockwise to unlock it from the base plate.
- Take the dishes into a laminar flow cabinet and aseptically remove the coverslips. Transfer them to sterile Petri dishes containing fresh medium or phosphate-buffered saline (PBS) for further inspection and evaluation.
6. Evaluation of the Effect of TTFields
NOTE: TTFields' effects can be determined in several ways. Use one or more of the following methods to compare treated cells to untreated control cells:
- Cell count
- Remove the medium/PBS from each coverslip-containing dish.
- Add 0.5 mL of 0.25% trypsin/EDTA to each dish and incubate for up to 10 min (i.e., until the cells start to detach from the coverslip surface) at 37 °C in a CO2 incubator.
- Add 0.5 mL of the complete growth medium to neutralize the trypsin and re-suspend the cells by gently pipetting up and down.
- Count the cells using any standard cell counting technique that is compatible with counting low cell concentrations (i.e., around 20,000 cells/mL).
- Clonogenic assay
- Plate a similar number of cells (100-500 cells per dish) from each dish onto new, sterile Petri dishes or 6-well plates containing 2 mL of fresh complete growth medium.
- Incubate in a CO2 incubator at 37 °C for 1-3 weeks (depending on the properties of each cell line) until colonies comprised of ~50 cells are formed. Evaluate the cell number per colony by light microscopy.
- Remove the medium and rinse with PBS.
- Remove the PBS and add ice-cold methanol (1 mL). Incubate at -20 °C for 10 min or longer.
- Remove the methanol.
- Add crystal violet solution (0.1% w/v in 25% v/v methanol in water) and incubate for 20 min.
CAUTION: Crystal violet is a potential carcinogen; use gloves while working with crystal violet. - Remove the crystal violet, wash 3 times with deionized water, and air-dry.
- Count the colonies formed in each dish.
Representative Results
Outcomes after TTFields application when scanning different frequencies can be quantified based on different assays, such as cell counts, colorimetric assays, clonogenic assays, and examinations on the changes in the number of invading cells using a Boyden chamber placed inside a special high-wall TTFields cell culture dish. Careful experimental planning based on predetermined cell doubling times will allow the cells to reach the maximal number of mitotic events during the treatment duration, thus maximizing treatment outcomes.
Figure 2 illustrates a typical frequency scan outcome for an average cell count (i.e., number of cells) and clonogenic assay of A2780 (i.e., human ovarian cancer cells; Figure 2A)7 and F-98 (i.e., rat glioma cells; Figure 2B)1 treated with two directional TTFields (frequency range: 100-500 kHz, RMS: 1.7 V/cm, and incubator temperature: 18 °C). The results demonstrate a significant reduction in the cell counts at all applied frequencies, with the maximal reduction at 200 kHz for all cell lines tested (one-way ANOVA with multiple comparison). The optimal frequency leading to the highest reduction in the clonogenic potential was the same for both A2780 and F98 cells (Figures 2B and C). The Pearson correlation coefficient between the cell number and the clonogenic effect at each frequency was 0.967 (p = 0.002) and 0.755 (p = 0.083) for A2780 and F98, respectively.
Figure 3 demonstrates a frequency scan outcome for the average cell count for OVCAR-3 (i.e., human ovarian cancer cells; Figure 3A) and U-87 MG (i.e., human glioma cells; Figure 3B) cells treated with two directions of TTFields at different intensities (RMS: 1.7, 1.3, and 1.0 V/cm, respectively and incubator temperature: 18 °C, 24 °C, and 28 °C, respectively). The results demonstrate that while there is still a significant reduction in OVCAR-3 cell count after treatment with 1.3-V/cm TTFields (incubator temperature: 24 °C), the effect is relatively small compared to the results obtained when the cells were treated with 1.7 V/cm (incubator temperature: 18 °C). U-87 MG cells treated with 1.0-V/cm TTFields (incubator temperature: 28 °C) also demonstrate a similar trend in the reduction of cell number as when treated with 1.7 V/cm, yet the effect was not significant at lower intensities.

Figure 1. TTFields dishes were installed onto a base plate unit and connected to the flat TTFields control cable. Please click here to view a larger version of this figure.
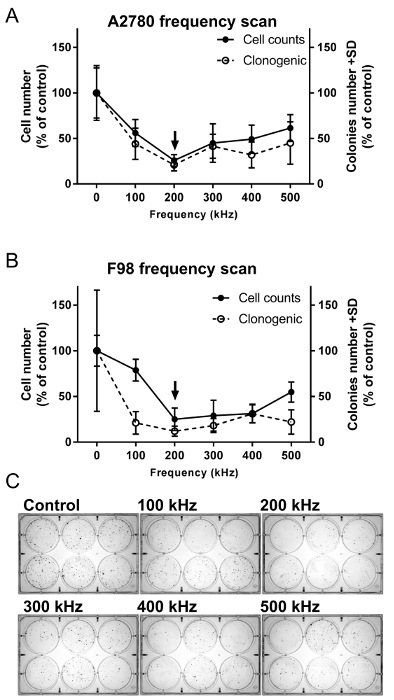
Figure 2. Frequency scans for the determination of the optimal TTFields inhibitory frequency for (A) A2780 and (B) F98 cells. Cells were treated for 72 h with TTFields of different frequencies (1.7 V/cm and 100-500 kHz). The effect of TTFields treatment was estimated using cell count and clonogenic assays. The arrow indicates the optimal frequency. The results represent averages ± SD based on at least 6 replicates for each frequency tested. (C) Representative images of the clonogenic survival of A2780 cells following TTFields treatment at various frequencies. Please click here to view a larger version of this figure.
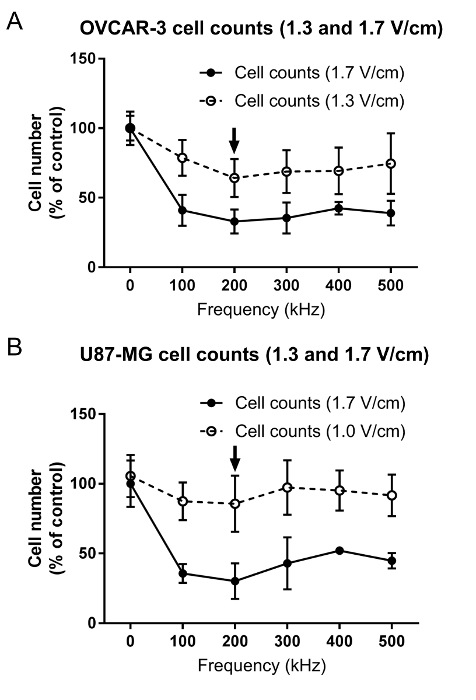
Figure 3. Frequency scans for the determination of the optimal TTFields inhibitory frequency for (A) OVCAR-3 and (B) U-87 MG cells. Cells were treated for 72 h with TTFields of different frequencies (100-500 kHz) and intensities (OVCAR-3: 1.3 and 1.7 V/cm; U-87 MG: 1.0 and 1.7 V/cm). The effect of TTFields treatment was estimated using cell counts. The arrow indicates the optimal frequency. The results represent averages ± SD based on at least 6 replicates in each frequency tested. Please click here to view a larger version of this figure.
| Incubator ambient temperature | Expected TTFields intensities |
| (°C) | (V/cm RMS) |
| 18 | 1.62 |
| 19 | 1.55 |
| 20 | 1.48 |
| 21 | 1.41 |
| 22 | 1.33 |
| 23 | 1.26 |
| 24 | 1.19 |
| 25 | 1.12 |
| 26 | 1.04 |
| 27 | 0.97 |
| 28 | 0.9 |
| 29 | 0.83 |
| 30 | 0.76 |
Table 1. Incubator ambient temperature and expected TTFields intensities inside the TTFields dish.
Discussion
TTFields are an emerging anti-tumor modality based on the continuous application of properly tuned alternating electric fields1,2,8,9,10,17. Maximizing anti-tumor efficacy is a desirable outcome for all treatment modalities. Thus, "fighting" for every additional percent of cancerous cell growth inhibition may have a significant effect on the long-term clinical outcome for patients. This is because of the required continuous nature of TTFields application and the resulting cumulative effect. Maximizing TTFields application can be achieved in several ways: (1) increasing the electric field intensity1,7, (2) lengthening treatment duration5, (3) finding the most effective combination with other treatment modalities18,19, and (4) defining the optimal frequency1,2,4,6,7. Maximizing the electric field intensity at the site of the tumor is achieved by optimizing the location of the arrays on the patient skin; this allows for the delivery of the maximal field intensity to the tumor based on the individual anatomy of the patient20. Lengthening treatment duration mostly relies on patient compliance with treatment (for at least 18 h per day)17. Finding the right combination with other therapies and determining the optimal frequency relies heavily on in vitro results, as no validated markers for TTFields treatment outcomes are currently available. In this work, we have outlined the experimental procedures required to determine the optimal TTFields frequency for cancerous cell lines using the TTFields in vitro application system. The methods described here can potentially be used to screen the combination of other cancer treatment modalities (e.g., chemotherapy agents or irradiation) with TTFields and to determine the optimal frequency for TTFields administration for each specific combined treatment.
In line with previous publications, the results shown here demonstrate that the optimal frequency for the treatment of both glioma cells and ovarian cancer cells is 200 kHz1,7. In this work, we demonstrated for the first time that the optimal TTFields frequency to reduce the clonogenic potential is associated with the frequency that leads to the maximal cytotoxic effect. The methods used in this work to quantify the effects of TTFields (i.e., cytotoxic and clonogenic) are only two of many possible standard endpoint assays to evaluate treatment outcomes. Additional treatment outcome tests include: (1) fixing, staining, and mounting the coverslips on which the cells are plated over a microscope for the visualization of intracellular structures; (2) performing assays of protein and RNA extracts, either from the TTFields dishes themselves or after transferring the coverslip to a new disposable dish; and (3) trypsinizing cells stained for flow cytometry analysis.
Careful experimental planning will impact the treatment outcomes after the delivery of TTFields. The key steps include ensuring that the cell proliferation throughout the experiment does not lead to overgrowth and using the appropriate electric field intensity, as intensities that are too high when applied to sensitive cell lines will result in too-few cells for the required assays to determine the optimal frequency. Conversely, TTFields applied at very low intensities on less sensitive cell lines will result in small effects that may be masked by inherent variation. The treatment logs should be examined for valuable information regarding temperature stability, electrical currents, and resistance for each and every dish throughout the experiment. Replacing faulty dishes at treatment start and excluding data from a dish that did not meet the desirable treatment parameters will minimize variability between replicates.
In summary, TTFields are an emerging anticancer treatment modality that has already demonstrated efficacy and safety in clinical settings8,9,10. Testing TTFields in an in vitro setting using the protocols described here may allow for the optimization of TTFields treatment parameters in the clinical setting and may broaden our understanding of the underlying mechanism of action.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Authors have no acknowledgements.
Materials
| inovitro system and software | Novocure | ITG1000 and IBP1000 | Each unit contains 1 TTFields generator, 1 base plate, 8 TTFields dishes with covers and 1 flat cable. |
| Sterilization bags | Westfield medical | 24882 | |
| Plastic cover slides | Thermo Scientific (NUNC) | 174977 | Pre treated and sterilized |
| Glass cover slides | Thermo Scientific (Menzel-Gläser) | CB00220RA1 | Sterilize if necessary |
| Dulbecco’s modified Eagle’s medium | Biological Industries (Israel) | 01-055-1A | Warm in 37 °C water bath before use |
| RPMI 1640 | Gibco | 21875-034 | Warm in 37 °C water bath before use |
| Fetal Bovine Serum (FBS) | Biological Industries (Israel) | 04-007-1A | Warm in 37 °C water bath before use |
| L-Glutamine 200mM (100X) | Gibco | 25030-029 | |
| Pen/Strep (10000 U/mL Penicillin, 10000 µg/mL Streptomycin) | Gibco | 15140-122 | |
| Sodium Pyruvate solution 100 mM | Biological Industries (Israel) | 03-042-1B | |
| Hepes buffer 1M | Biological Industries (Israel) | 03-025-1B | |
| Insuline solution from bovine pancreas | Sigma-Aldrich | 10516-5ML | |
| 0.25% Trypsin/EDTA | Biological Industries (Israel) | 03-050-1B | Warm in 37 °C water bath before use |
| Methanol | Merck | 1.06009.2511 | Cool to -20 °C in the freezer before use |
| Crystal violet | Sigma-Aldrich | 120M1445 | Harmful. Prepare 0.1% w/v crystal violet solution in 25% Methanol 75% water. |
| Light detergent | Alcononx | 242985 | Prepare 5% solution in water, or according to manufacurer's instrutions. |
| PBS | Biological Industries (Israel) | 02-023-1A | Without calcium and magnesium |
| A2780 | ECACC | 93112519 | Grow in RPMI 1640 supplemented with FBS (10%), pen/strep (100 U/mL / 100 µg/Ml), sodium pyruvate (1 mM) and Hepes buffer (12mM). |
| F98 | ATCC | CRL-2397 | Grow in Dulbecco’s modified Eagle’s medium supplemented with FBS (10%), pen/strep (100 U/mL / 100 µg/Ml), sodium pyruvate(1 mM) and glutamine (2mM). |
| Ovcar-3 | ATCC | HTB-161 | Grow in RPMI 1640 supplemented with FBS (20%), pen/strep (100 U/mL / 100 µg/Ml), sodium pyruvate (1 mM), Hepes buffer (12 mM) and insuline (10 µg/mL). |
| U-87 MG | ATCC | HTB-14 | Grow in Dulbecco’s modified Eagle’s medium supplemented with FBS (10%), pen/strep (100 U/mL / 100 µg/Ml), sodium pyruvate(1 mM) and glutamine (2mM). |
| refrigirated CO2 incubator | CARON | 7404-10-3 | |
| Laminar flow cabinet | ADS Laminair | Bio12 and VSM12 |
Riferimenti
- Kirson, E. D., et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 104 (24), 10152-10157 (2007).
- Kirson, E. D., et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 64 (9), 3288-3295 (2004).
- Gera, N., et al. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One. 10 (5), (2015).
- Giladi, M., et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology. 14 (1), 54-63 (2014).
- Giladi, M., et al. Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci Rep. 5, 18046 (2015).
- Giladi, M., et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin Oncol. 41, S35-S41 (2014).
- Voloshin, T., et al. Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int J Cancer. 139 (12), 2850-2858 (2016).
- Pless, M., Droege, C., von Moos, R., Salzberg, M., Betticher &, D. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer. 81 (3), 445-450 (2013).
- Stupp, R., et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA. 314 (23), 2535-2543 (2015).
- Stupp, R., et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 48 (14), 2192-2202 (2012).
- Kanner, A. A., et al. Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A system versus best physician’s choice chemotherapy. Semin Oncol. 41, S25-S34 (2014).
- Miranda, P. C., Mekonnen, A., Salvador, R., Basser &, J. P. Predicting the electric field distribution in the brain for the treatment of glioblastoma. Phys Med Biol. 59 (15), 4137-4147 (2014).
- Wong, E. T., Lok, E., Swanson &, D. K. An Evidence-Based Review of Alternating Electric Fields Therapy for Malignant Gliomas. Curr Treat Options Oncol. 16 (8), (2015).
- Kim, E. H., et al. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. , (2016).
- Kim, E. H., Song, H. S., Yoo, S. H., Yoon &, M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. , (2016).
- Pavesi, A., et al. Engineering a 3D microfluidic culture platform for tumor-treating field application. Sci Rep. 6, 26584 (2016).
- Wong, E. T., Lok, E., Swanson &, D. K. Clinical benefit in recurrent glioblastoma from adjuvant NovoTTF-100A and TCCC after temozolomide and bevacizumab failure: a preliminary observation. Cancer Med. , (2015).
- Kirson, E. D., et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 9, (2009).
- Schneiderman, R. S., Shmueli, E., Kirson, E. D., Palti &, Y. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 10, 229 (2010).
- Connelly, J., et al. Planning TTFields treatment using the NovoTAL system-clinical case series beyond the use of MRI contrast enhancement. BMC Cancer. 16 (1), (2016).

