Using In Vivo and Tissue and Cell Explant Approaches to Study the Morphogenesis and Pathogenesis of the Embryonic and Perinatal Aorta
Summary
Protocols for studying the embryonic and perinatal murine aorta using in vivo clonal analysis and fate mapping, aortic explants, and isolated smooth muscle cells are detailed here. These diverse approaches facilitate the investigation of the morphogenesis of the embryonic and perinatal aorta in normal development and the pathogenesis in disease.
Abstract
The aorta is the largest artery in the body. The aortic wall is composed of an inner layer of endothelial cells, a middle layer of alternating elastic lamellae and smooth muscle cells (SMCs), and an outer layer of fibroblasts and extracellular matrix. In contrast to the widespread study of pathological models (e.g., atherosclerosis) in the adult aorta, much less is known about the embryonic and perinatal aorta. Here, we focus on SMCs and provide protocols for the analysis of the morphogenesis and pathogenesis of embryonic and perinatal aortic SMCs in normal development and disease. Specifically, the four protocols included are: i) in vivo embryonic fate mapping and clonal analysis; ii) explant embryonic aorta culture; iii) SMC isolation from the perinatal aorta; and iv) subcutaneous osmotic mini-pump placement in pregnant (or non-pregnant) mice. Thus, these approaches facilitate the investigation of the origin(s), fate, and clonal architecture of SMCs in the aorta in vivo. They allow for modulating embryonic aorta morphogenesis in utero by continuous exposure to pharmacological agents. In addition, isolated aortic tissue explants or aortic SMCs can be used to gain insights into the role of specific gene targets during fundamental processes such as muscularization, proliferation, and migration. These hypothesis-generating experiments on isolated SMCs and the explanted aorta can then be assessed in the in vivo context through pharmacological and genetic approaches.
Introduction
The circulatory systems of multicellular organisms function to deliver nutrients and oxygen to cells that are not in contact with the external environment and to remove waste products and carbon dioxide from these cells. In vertebrates, the primary circulatory system consists of the heart, which pumps blood through a series of blood vessels. The walls of large blood vessels, such as arteries and veins, consist of three layers: i) the intima, or inner layer of endothelial cells; ii) the media, or middle layer of alternating circumferentially elongated smooth muscle cells SMCs and elastic lamellae; and iii) the adventitia, or outer layer of connective tissue and fibroblasts. The vast majority of studies in vascular biology focus on endothelial cells, investigating the formation of new endothelial cell-lined tubes through angiogenesis. In comparison, SMCs receive relatively little attention. However, SMCs are a critical cell type in the construction of the normal arterial wall and in vascular pathologies.
The aorta is the largest-caliber artery in the body, receiving the cardiac output from the left ventricle of the heart. It is afflicted by diverse human diseases, including atherosclerosis, aneurysm, and dissection. In adult organisms, the aorta and its major branches are intensely studied in models of vascular disease. For instance, high fat diet fed mice that are null for the gene encoding the low-density lipoprotein receptor or apolipoprotein E, develop atherosclerosis, and recent fate mapping studies indicate that pre-existing SMCs give rise to multiple cell types in the atherosclerotic plaque1. In aortic aneurysms, pathological changes include SMC apoptosis and extracellular matrix remodeling2,3.
Substantially less is known regarding SMC morphogenesis and pathogenesis during the embryonic and perinatal periods. Here, we provide protocols for studying embryonic and perinatal aortic SMCs in vivo, in tissue explants and in isolated cells. For instance, the first section of the protocol delineates fate mapping and clonal analysis in embryonic mice. Cre recombinase expressed under the control of a cell-specific promoter facilitates the marking of specific cells and their progeny4,5,6; however, temporal control of cell-specific labeling can be challenging during embryonic development in mice. In this context, with embryos expressing the conditional CreER under a promoter active in SMCs (e.g.,Myh11 or Acta2) and a Cre reporter, we provide methods for injecting tamoxifen or its active metabolite 4-OH-tamoxifen in pregnant dams and for analyzing the labeled cells in embryos or postnatal offspring. Furthermore, in contrast to fate mapping studies, which predominately utilize Cre reporters with a single reporter fluorophore1,7, clonal analysis is substantially enhanced with multi-color Cre reporters.
The second and third sections of protocol describe methods for isolating and culturing embryonic aortic explants and aortic SMCs from neonates, respectively. These approaches allow for the manipulation of signaling pathways, specifically in aortic explants or SMCs, and for analyzing the direct effects of pharmacological agents. Thus, the role of specific genes in the tissue of interest can be screened in a far more rapid fashion than through traditional genetic manipulations in mice. In addition, the isolated SMC studies facilitate the analysis of cell migration and adhesion, which are technically limited in vivo.
Finally, the fourth protocol section delineates the placement of a subcutaneous osmotic mini-pump loaded with pharmacological agents in pregnant (or non-pregnant) mice. This method facilitates the analysis of the effect on embryonic development caused by agents that require continuous infusion because of rapid metabolism. The alternative of frequent injections is not practical for many agents and should be avoided, as it may cause significant discomfort in the pregnant dam.
Protocol
All mouse protocols are approved by the Institutional Animal Care and Use Committee at Yale University.
1. In Vivo Embryonic Fate Mapping and Clonal Analysis
Note: We have used these approaches widely to evaluate the origins of cells and their clonal architecture in development and disease models7,8,9,10.
- Set up mating between mice with a CreER and mice with a Cre reporter.
NOTE: A CreER is used for SMC marking; Myh11-CreERT2 or Acta2-CreERT2 mice11,12 is commonly used for this purpose. We have used ROSA26R-CreERT2 mice13 for broadly marking embryonic tissues.- For clonal analysis, use a multi-color Cre reporter (e.g., Confetti or Rainbow [Rb] mice)8,14,15 and for fate mapping, use a single-color Cre reporter (e.g., ROSA26R-YFP mice16) or the double-color Cre reporter ROSA26R-mTomato-mGFP (mTmG)17.
NOTE: This breeding scheme uses adult mice that are 2 – 6 months old (generally ~30 g) and will generate embryos with a CreER and a Cre reporter.
- For clonal analysis, use a multi-color Cre reporter (e.g., Confetti or Rainbow [Rb] mice)8,14,15 and for fate mapping, use a single-color Cre reporter (e.g., ROSA26R-YFP mice16) or the double-color Cre reporter ROSA26R-mTomato-mGFP (mTmG)17.
- Check for vaginal plugs in the morning using a metal probe and consider noon on the day of plug detection as embryonic day (E) 0.5.
- Separate the female from the male when a plug is detected.
- Prepare 4-OH-tamoxifen and tamoxifen working solutions.
- For 4-OH-tamoxifen, dissolve 5 mg into 50 µL of 100% ethanol, vortex for 2 min and then add 450 µL of corn oil. On ice, sonicate at output level 2 for 10 s cycles with resting between cycles until sample cools down. After sample is completely dissolved (usually ~ 4 – 5 cycles of sonication), take 200 µL of sonicated solution and dissolve in 1,800 µL of corn oil to make final working solution (4-OH-tamoxifen, 1 mg/mL).
- For tamoxifen, dilute to 50 mg/mL in 100% ethanol and vortex until it is dissolved. Dilute in corn oil to make the final working solution (tamoxifen, 10 mg/mL) and stir at 45 °C to ensure that it is completely dissolved.
- For early embryonic induction, inject 4-OH-tamoxifen intraperitoneally into a pregnant dam.
- Use 4-OH-tamoxifen injections (up to ~150 µg per pregnant mouse, or roughly 5 mg/kg bodyweight) at E5.5 and thereafter as they yield viable dam, embryos, and pups.
- Use tamoxifen at doses of 0.5 – 1.5 mg (or 17 – 50 mg/kg) for dams pregnant with embryos at ~E9 and thereafter. Use a 30G needle for all injections.
Note: Filtration through a 0.22 µm filter is typically used to sterilize all mixtures prior to injection.
- For clonal analysis, use intraperitoneal injections of high doses of 4-OH-tamoxifen (e.g., 5 mg/kg) or tamoxifen (e.g., 50 mg/kg) to mark multiple cells.
- To mark individual cells, titrate down the 4-OH-tamoxifen or tamoxifen dose such that in the tissue of interest (i.e., the aorta), almost all embryos analyzed (as described below at the end of this section) have either no cells labeled or only cells of a single color; this is the threshold dose.
- To mark cells in the mid-late gestational period and to trace their fate or clonality in the postnatal mouse, inject progesterone into the intraperitoneal cavity of the pregnant dam concomitantly with tamoxifen in a 1:2 (progesterone:tamoxifen) dose ratio.
NOTE: Tamoxifen doses are 17 – 50 mg/kg and progesterone doses are 8.5 – 25 mg/kg (i.e., half of the tamoxifen doses). The progesterone injection may delay labor by ~1 – 2 days. - Euthanize the dam pregnant with embryos of a desired age using an open drop method, where the dam is placed in a receptacle containing cotton or gauze soaked with undiluted isoflurane. Confirm death by opening the chest cavity to induce pneumothorax, by removing the vital organs, and/or by performing cervical dislocation.
- Cleanse the abdomen with 70% ethanol. Use scissors to cut open the abdomen and dissect out the uterus. Remove the embryos from uterus and carefully separate the embryos from the placenta and yolk sac using forceps.
- Euthanize the embryos at E15 or older by performing either cervical dislocation or decapitation with surgical scissors or a sharp blade.
NOTE: Embryos younger than E15 rapidly die following euthanasia of the mother and/or the removal of the embryos from mother. - Place the embryos in ice-cold PBS and cut a small piece of tail using scissors to genotype for the CreER and Cre reporter.
- Fix the embryos in 4% paraformaldehyde at 4 °C for 2 h. Wash the embryos three times in PBS and then incubate the embryos in 30% sucrose in PBS in 15-mL tubes, waiting until they sink, which may take up to two days.
- Fill plastic freezing molds up to 2/3 of maximum level with optimum cutting temperature (OCT) compound. Place each embryo vertically in the mold, entirely submerged in OCT. Incubate for 10 min at room temperature (RT) to minimize bubbles.
- Place freezing tissue blocks upright in a dry ice, 70% ethanol bath to freeze. Store the blocks at -80 °C.
- Set the cryostat temperature to -22 °C and cut blocks to generate transverse sections through the entire aorta, 10 – 20 µm thick. Dry the slides at RT for 30 min prior to storing them at -80 °C.
- Thaw the slides at RT, protected from light to avoid the bleaching of fluorophores. Wash the slides with PBS-Tween20 0.1%.
- For clonal analysis, stain the slides for nuclei (DAPI, 5 mg/mL) and directly image other fluorophores in the Rainbow Cre reporter (Cerulean: 433-nm excitation max, 475-nm emission max; mOrange: 548 nm, 562 nm; mCherry: 587 nm, 610 nm).
- Use the following filters for detecting fluorophores with an upright fluorescent microscope: DAPI (excitation max/bandwidth 350/50 nm, emission max/bandwith 460/50 nm), Cerulean (excitation 436/20 nm, emission 480/40 nm), texas red (excitation 560/40 nm, emission 630/75 nm) and custom filter to separate mCherry from mOrange (excitation 577/10 nm, emission 630/50 nm).
- For fate mapping with the mTmG Cre reporter, detect GFP (484 nm, 510 nm), either by directly imaging or by using immunostaining. For imaging with an upright fluorescent microscope use a GFP filter (excitation 470/40 nm, emission 525/50 nm).
- For immunostaining, incubate sections with primary antibodies overnight at 4 °C, wash with 0.1% Triton X-100 in PBS and then incubate with secondary antibodies for 1 h. Use anti-CD31 (final concentration 0.0016 mg/mL), anti-GFP (0.006 mg/mL) and Cy3-directly conjugated anti-SMA (1:500 final dilution) primary antibodies and use secondary antibodies directly conjugated to fluorophores (1:500 final dilution) (see the Table of Materials).
- For clonal analysis, stain the slides for nuclei (DAPI, 5 mg/mL) and directly image other fluorophores in the Rainbow Cre reporter (Cerulean: 433-nm excitation max, 475-nm emission max; mOrange: 548 nm, 562 nm; mCherry: 587 nm, 610 nm).
- Image slides with an upright fluorescent microscope (magnification: 4X – 20X) or confocal microscope (10X -63X). For high magnification, use a confocal imaging 63x oil objective with a numerical aperture of 1.4-0.6 and a frame size of 1,024 x 1,024 pixels.
2. Explant Embryonic Aorta Culture
Note: This approach was previously used to evaluate the role of integrin beta3 in stenosis of the explanted Eln(-/-) embryonic aorta9,18.
- Euthanize a timed-pregnant dam at E15.5 by excess isoflurane inhalation, as per step 1.8, above.
- Harvest and euthanize the embryos, as per steps 1.9 – 1.10, above.
- Position the embryo supinely and pin the extended limbs to the dissection board. Visualize with a dissection stereoscope and use scissors to cut a vertical incision from the abdomen through the sternum to the upper thorax. Dissect away the thymus, trachea, lungs, esophagus, liver, and intestine.
- Gently pull the heart ventrally using forceps and use scissors to dissect the aorta away from the dorsal aspect of the thoracic and abdominal cavities. To release the aorta, cut it at the proximal root and the distal abdominal positions.
- Place the aorta in ice-cold sterile PBS in a cell culture hood. Transfer the aorta to a 24-well plate and culture it in DMEM with 0.5% FBS for up to 24 h at 37 °C.
NOTE: The culture medium can be supplemented with a blocking antibody (e.g., anti-integrin αvβ3 antibody at 0.02 mg/mL9; see the Table of Materials) or a pharmacological agent. - Wash the aorta twice with PBS and then fix it with 4% paraformaldehyde for 20 min.
- Wash three times with PBS and transfer the aorta to a 1.5-mL tube with 30% sucrose in PBS. After the aorta sinks, make frozen tissue blocks, cut sections, and immunostain, as described in steps 1.13 – 1.15, above.
3. SMC Isolation from the Perinatal Aorta
Note: This approach is currently being used to compare the biology of aortic SMCs isolated from Eln(-/-) and wildtype perinatal mice.
- Euthanize a murine pup at postnatal day (P) 0.5, either by cervical dislocation or by decapitation with surgical scissors or a sharp blade, as described in step 1.10, above.
- Perform step 2.3 and then, after opening the thorax and abdomen, puncture left ventricle with 23G needle. Use plastic tubing to connect the needle to sterile PBS-containing syringe held vertically at an elevated position so that PBS flows into the left ventricle by gravity. Allow infusion of sterile PBS into left ventricle until the liver blanches.
- Use forceps to remove adventitial tissue on the outside of the aorta and dissect the aorta as described in step 2.4.
- Digest the aorta in a single solution of DMEM (500 µL) supplemented with 225 U/mL collagenase, 2.25 U/mL elastase, and 1x antibiotic-antimycotic for 45 min at 37 °C. Manually shake the tube every 5 – 10 min.
- In the sterile cell culture hood, titurate the digested tissue by pipetting up and down with a 200-µL pipette to generate a single-cell suspension. Transfer the single-cell suspension to a 15-mL tube, add 2 mL of DMEM, and centrifuge at 920 x g for 5 min at 25°C.
- Discard the supernatant. Resuspend the cell pellet in 3 mL of SMC culture medium (DMEM containing 10% FBS supplemented with 1 µg/mL rhFGF, 10 µg/mL rhEGF, 100 U/mL penicillin/streptomycin, and 2.5 µg/mL Amphotericin B). Transfer to a 35-mm culture plate.
NOTE: Upon initial isolation, ~400,000 cells are obtained from a single P0.5 wildtype aorta; ~300,000 of these cells are SMCs. - Culture the cells at 37 °C and change to fresh SMC culture medium every 3 days. Using standard techniques with trypsin, passage the cells when confluent. For experiments, use cells from passages 3 – 7.
4. Subcutaneous Osmotic Mini-pump Placement in Pregnant (or Non-pregnant) Mice
Note: This approach was previously used to continuously deliver a pharmacological agent (e.g., integrin β3 and β5 inhibitor cilengitide) to embryos in utero9. The pump was inserted in the pregnant dam at E13.5 and maintained until parturition.
- Anesthetize the mice with 2 – 5% isoflurane in oxygen (flow rate of 1 L/min) for induction and 1-3% for maintenance. Use the toe-pinch reflex to monitor the level of anesthesia, and adjust the anesthetic agent as needed. Administer subcutaneous buprenorphine (0.05 – 0.1 mg/kg) for analgesia.
- Shave the lower back with clippers. Use sterile drapes, gloves, and instruments and maintain the mice on a heating surgical plate during surgery.
- Place each mouse in prone position and scrub the back with betadine followed by isopropyl alcohol. Approximately 3 – 5 cm rostral to the base of the tail in the lower back, use a scalpel to make a horizontal skin incision 0.5 – 1.0 cm in length without injuring the underlying muscle.
- Insert a hemostat into the incision and create a subcutaneous pocket rostrally for pump implantation by opening and closing hemostat jaws.
- Fill an osmotic mini-pump with the agent of choice, as per the manufacturer's guidelines (see the Table of Materials). Insert the filled pump into the pocket and close the incision with clips or 6-0 non-absorbable sutures. Ensure that the total surgical time is less than 10 min.
- Post-operatively, monitor the mice every 15 min until they have recovered from sternal recumbency. Administer subcutaneous buprenorphine (0.05 – 0.1 mg/kg) every 6 – 12 h for 48 h. When the mice have fully recovered from general anesthesia, monitor them daily. Remove the clips or sutures 7 – 10 days post-surgery.
Representative Results
In a representative clonal analysis of SMCs in embryos mutant for Eln (the gene encoding the extracellular matrix protein elastin), Eln(+/-), Acta2-CreERT2 mice were mated to Eln(+/-) mice also carrying the multi-color ROSA26R(Rb/Rb) reporter. As described in step 1, plugs were checked, pregnant dams were induced with a single tamoxifen injection (1.5 mg) at E12.5, and they were sacrificed at E18.5. Embryos were harvested, fixed, frozen, and genotyped. Transverse descending aortic cryosections were cut. Sections from Eln(+/-), Acta2-CreERT2, ROSA26R(Rb/+) embryos indicate that the excess inner layer SMCs that accumulate in Eln-null mice (starting after E15.5) are marked by multiple colors (Figure 1) and hence derive from multiple alpha-smooth muscle actin (SMA)+ cells that are present at ~E12.5. In the E12.5 aorta, SMA+ cells are limited to the tunica media.
To generate Eln(-/-) embryonic aortas for explants, male and female Eln(+/-) mice were mated. Using methods described in step 2, plugs were checked and pregnant Eln(+/-) dams were sacrificed at E15.5. The aortas were isolated from Eln(-/-) embryos and cultured for 0 or 18 h in the presence of an anti-integrin β3 blocking antibody or an IgG1 control. Aortas were then fixed, frozen, and cryosectioned, and cryosections were stained for SMA (SMC marker), CD31 (endothelial cell marker), and nuclei (DAPI) (Figure 2). The results demonstrate that within 18 h of culturing, the E15.5 Eln(-/-) aorta becomes hypermuscular and stenotic. This process is attenuated by an anti-integrin β3 blocking antibody.
SMCs were isolated from the aorta of wildtype mice at P0.5. As described in step 3, SMCs were isolated from the dissected neonatal aorta by enzyme digestion and were cultured. Cells were passaged, and passage 3 cells were stained for SMC markers (i.e., SMA, smooth muscle myosin heavy chain (SMMHC), or transgelin (also known as SM22α)), CD31, and nuclei (DAPI) (Figure 3). Most of the cultured cells express SMC markers but not CD31.
To evaluate whether pharmacological inhibition of integrin β3 can attenuate hypermuscularization and stenosis of the Eln(-/-) aorta in vivo, we continuously infused the inhibitor cilengitide because of its short half-life in plasma. Eln(+/−) males and females were mated, and, as described in step 4, osmotic mini-pumps loaded with cilengitide were implanted in pregnant dams at E13.5. At P0.5, pups were euthanized and genotyped, and their aortas were analyzed. Cilengitide treatment significantly attenuates aortic stenosis and hypermuscularization in Eln(-/-) mice (Figure 4) without altering the wildtype aorta9. Thus, anti-integrin β3 is a potentially promising noninvasive approach to treat elastin aortopathy.
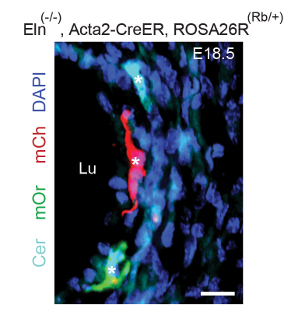
Figure 1. Excess SMCs in the Eln(-/-) Aorta Derive from Multiple Pre-existing SMCs. Dams pregnant with Eln(-/-), Acta2-CreERT2 embryos also carrying the multicolor Cre reporter ROSA26R(Rb/+) were induced with a single intraperitoneal injection of tamoxifen (1.5 mg) at E12.5. Dams were sacrificed at E18.5, and transverse sections of the descending aortas of the embryos were analyzed, with staining for DAPI and the direct fluorescence of Rb colors, Cerulean (Cer), mCherry (mCh), and mOrange (mOr). Excess SMCs accumulate in the inner aspect of the Eln-null aorta after E15.518. The excess inner-layer SMCs include cells of multiple colors (asterisks), indicating polyclonality. Lu, aortic lumen. Scale bar, 10 µm. Reprinted from Misra et al., 20169. Please click here to view a larger version of this figure.
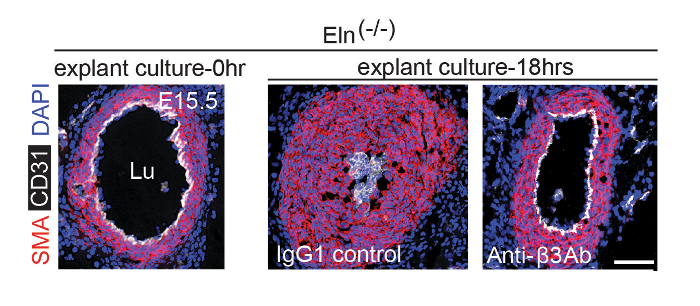
Figure 2. Anti-αvβ3 Integrin Blockade Attenuates Hypermuscularization and Stenosis of Eln(-/-) Aortic Explant. At E15.5, pregnant dams were euthanized and the aortas of Eln(-/-) embryos were harvested. Isolated aortas were either fixed immediately or cultured in the presence of an isotype control IgG1 or an integrin αvβ3 blocking antibody for 18 h prior to fixation. Fixed aortas were stained for CD31, SMA, and nuclei (DAPI). Lu, aortic lumen. Scale bar, 100 µm. Reprinted from Misra et al., 20169. Please click here to view a larger version of this figure.
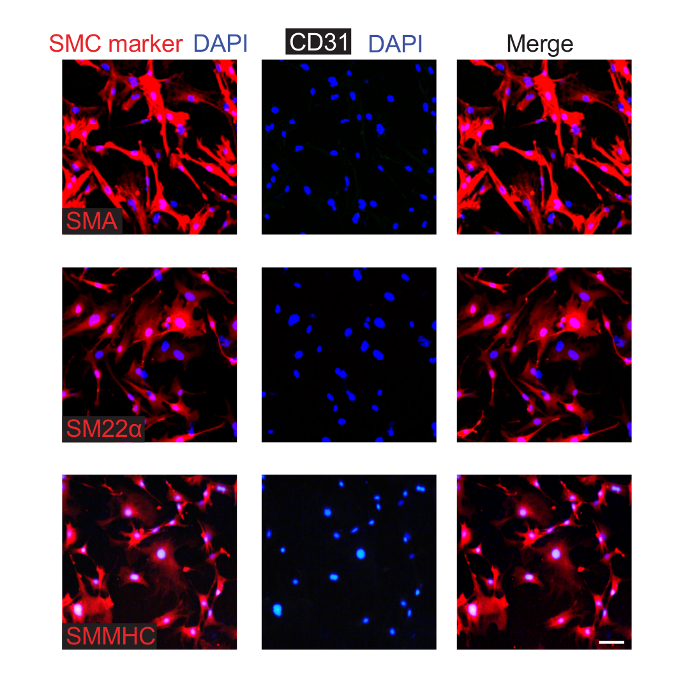
Figure 3. Isolated and Cultured Aortic SMCs from Neonatal Mice Express Smooth Muscle Markers. SMCs were isolated from P0.5 pups and were cultured. Cells from the third passage were fixed and stained for CD31 (EC marker), nuclei (DAPI), and an SMC marker (either SMA, SM22α, or SMMHC, as indicated). This analysis indicates that ~90% of the cultured cells express SMC markers, and none were observed to express CD31. Scale bar, 100 µm. Please click here to view a larger version of this figure.
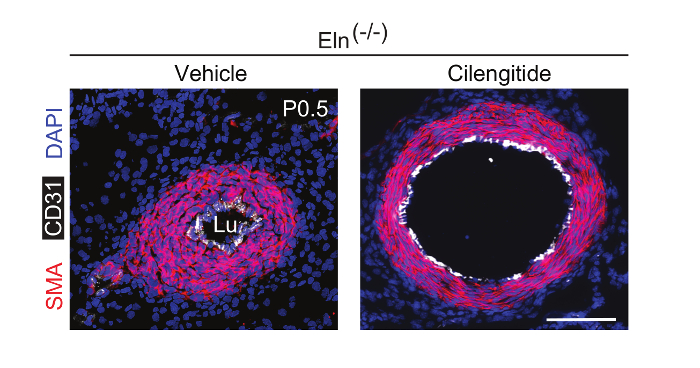
Figure 4. Continuous Infusion of Cilengitide In Vivo Attenuates Eln(-/-) Aortic Hypermuscularization and Stenosis. Male and female Eln(+/-) mice were crossed. The integrin β3 inhibitor cilengitide or vehicle (PBS) was continuously infused in pregnant dams using osmotic mini-pumps starting at E13.5. Transverse sections at P0.5 were stained for SMA (red), CD31 (white), and nuclei (DAPI, blue). Lu, aortic lumen. Scale bar, 100 µm. Please click here to view a larger version of this figure.
Discussion
In contrast to the extensive investigations of the murine aorta and its major branches in adult pathological conditions, such as models of atherosclerosis, less is known regarding the morphogenesis and the pathogenesis of the embryonic and perinatal aorta. Here, we focus on the embryonic/perinatal aorta, specifically the SMCs, and provide protocols to study the aorta through in vivo, tissue explant, and SMC isolation approaches. These complimentary approaches provide the investigator with diverse approaches to study the embryonic/perinatal aorta.
Clonal analysis is a very powerful approach that allows one to investigate the behavior of individual cells. We have recently used this approach to help identify specialized SMCs in the pulmonary vasculature10. It is critical to recognize that, in an individual mouse carrying a multi-color Cre reporter, there is a chance that cells of the same color could derive from more than one recombination event. Thus, clonal analysis with a multi-color reporter necessitates the evaluation of numerous mice. Because tamoxifen in the mid-late gestational period can interfere with parturition, for experiments that mark cells in the mid-late gestational period and trace them postnatally, progesterone and tamoxifen should be injected concomitantly. Similar to protocols in the embryo, fate mapping and clonal analysis of cells in the adult mouse can be undertaken with the intraperitoneal injection of tamoxifen in the adult.
Clonal analysis and fate mapping of SMCs uses mice carrying transgenes with the expression of Cre recombinase under the control of promoters with enhanced activity in the smooth muscle. Here, we describe protocols using mice carrying conditional transgenes Myh11-CreERT2 or Acta2-CreERT2. Myh11 is the most specific marker of SMCs19; however, it is downregulated in the Eln(-/-) aorta9. Acta2 is expressed in SMCs, including those of the Eln-null aorta, but it is not as specific because it is also expressed in other cell types. If these conditional transgenes were "leaky" (i.e., induce recombination in the absence of tamoxifen), it might be problematic, as the experiments would not delineate the timing of cell marking. The leakiness of these transgenes was analyzed by assessing beta-galactosidase activity in mice carrying a lacZ-based Cre reporter and either Myh11-CreERT2 or Acta2-CreERT2 following vehicle treatment (i.e., in the absence of tamoxifen induction)11,12. Based on this analysis, these transgenes were considered not leaky.
Hypermuscularization characterizes multiple vascular pathologies in humans. For instance, supravalvular aortic stenosis (SVAS) is a devastating congenital condition that is characterized by the obstruction of large and medium-sized arteries due to excess SMCs. SVAS results from the loss of function of one allele of the ELN gene and occurs as an isolated entity or, more commonly, as part of Williams-Beuren syndrome20,21,22,23,24. Eln(-/-) mutant mice phenocopy many aspects of the aortic phenotype of SVAS18,21,22. Aortic explants from Eln(-/-) embryos rapidly become occluded during culture, whereas explants from wildtype embryos remain patent9,18. The aortic explant approach facilitates screening for agents that attenuate stenosis in elastin aortopathy9. However, this approach does not take into account the mechanical or physical forces that the aorta is subject to while in the body. Thus, positive hits in aortic explant screening should be assessed with in vivo Eln mutant mouse models.
The SMC isolation protocol is a rapid and robust method to obtain SMCs from the perinatal murine aorta. A similar approach can be extrapolated to isolate SMCs from adult mice. Because of its relatively large size, the adult aorta can be opened longitudinally and the adventitia and endothelial cells dissected away. To evaluate the purity of the cells isolated from the perinatal or adult aorta, it is important to check for the expression of SMC markers (e.g., SMMHC, smoothelin, SM22α, and SMA) and the lack of expression of endothelial cell markers (e.g., CD31 and vascular-endothelial cadherin). A potential alternative isolation approach is to use flow cytometry to isolate fluorescently labeled SMCs from the dissected aorta. Mice carrying a fluorescent Cre reporter and Myh11-CreERT2, Acta2-CreERT2, or Tgln-Cre can be used to fluorescently label SMCs11,12,25,26,27. Alternatively, existing transgenic mice with the SMA promoter driving the expression of a fluorophore (i.e., GFP, mCherry, or RFP) can be used28,29,30.
Implanted osmotic mini-pumps are used to provide the continuous delivery of pharmacological agents to laboratory animals. We have implanted mini-pumps in pregnant mice at E13.5 to deliver agents to developing embryos during the rest of the gestational period9. To reach the embryos, such agents must be able to pass the blood-placental barrier. In general, mini-pumps are very well tolerated and, given the low rate of infection, routine antibiotics are not recommended. Wound dehiscence is rare; however, if a dehiscence greater than 4 mm is detected, the mouse should be re-anesthetized and the wound cleaned and closed again with a surgical suture.
This work describes an armamentarium of approaches that can be used to study the normal formation of the embryonic and perinatal aortic wall during development, as well as after perturbations in this process during disease. In addition, these protocols can be applied to study the maintenance and disease of the adult aorta. Approaches can be extrapolated to other blood vessels, as well as to smooth muscle-coated non-vascular structures, such as the gastrointestinal tract or the lung airways. Finally, similar techniques can be used to study cell types beyond SMCs, such as endothelial cells or fibroblasts, as well as the interplay between these cells types and SMCs.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Dean Li for sharing his laboratory’s protocol for aortic SMC isolation. Funding support was provided by the National Institutes of Health (R21NS088854, R01HL125815, and R01HL133016 to D.M.G), the American Heart Association (Grant-in-Aid 14GRNT19990019 to D.M.G.), and Yale University (Brown-Coxe Fellowship to A.M. and startup funds to D.M.G.).
Materials
| Tamoxifen | Sigma | T5648 | |
| Corn oil | Sigma | C-8267 | Vehicle for tamoxifen |
| 4-OH-tamoxifen | Sigma | H7904 | Active metabolite of tamoxifen |
| Progesterone | Sigma | P8783-5G | Use at half the concentration of tamoxifen |
| OCT compound | Sakura tissue tek | 4583 | For making cryoblocks |
| Cryomolds | Polysciences inc | 18986 | |
| DAPI | Sigma | D9542 | IHC staining of nucleus, final concentration 5 mg/ml |
| Cy3 directly conjugated anti-SMA antibody | Sigma | A2547 | IHC staining of SMA, final dilution 1:500 |
| Anti-CD31 antibody | BD Pharmingen | 550274 | IHC staining of GFP, final concentration 0.006 mg/ml |
| Anti-GFP antibody | Thermo Fisher Scientific | A-11121 | IHC staining of CD31, final concentration 0.0016 mg/ml |
| Secondary antibody goat anti-rabbit, Alexa 647 | Life Technologies | a21244 | IHC staining, final concentration 0.004 mg/ml |
| Secondary antibody goat anti-rabbit, Alexa 488 | Life Technologies | a11008 | IHC staining, final concentration 0.004 mg/ml |
| DMEM | Thermo Fisher Scientific | 10567-014 | For cell culture |
| FBS | Thermo Fisher Scientific | 10437028 | |
| Anti-integrin beta3 blocking antibody | BD Biosciences | 553343 | Clone 2C9.G2, final concentration 0.02 mg/ml |
| Collagenase | Worthington Biochemical Corp | 44H14977A | For digesting aorta |
| Elastase | Worthington Biochemical Corp | 34K15139 | For digesting aorta |
| Antibiotic-antimycotic (100X) | Thermo Fisher Scientific | 15240062 | |
| Recombinant human FGF | Promega | G5071 | |
| Recombinant human EGF | Promega | G5021 | |
| Penicillin/streptomycin (10,000 U/ml) | Thermo Fisher Scientific | 15140122 | |
| Amphotericin B | Thermo Fisher Scientific | 15290026 | |
| Tissue culture plates | Corning | CLS430165 | |
| Alzet osmotic mini-pump | Durect Corporation | 2001 | |
| ECLIPSE 80i Upright Fluorescent Microscope | Nikon | ||
| TCS SP5 | Leica | ||
| Branson Sonifier 450 | VWR | ||
| Myh11-CreERT2 mice | The Jackson Laboratory | 19079 | |
| Acta2-CreERT2 mice | Obtained from lab of Dr. Pierre Chambon and Daniel Metzger | ||
| ROSA26R-CreERT2 mice | The Jackson Laboratory | 8463 | |
| ROSA26R(mTmG/mTmG) mice | The Jackson Laboratory | 026862 | |
| ROSA26R(EYFP/EYFP) mice | The Jackson Laboratory | 006148 | |
| ROSA26R(Confetti/Confetti) mice | The Jackson Laboratory | 13731 | |
| ROSA26R(Rb/Rb) mice | Lab of Dr. Irv Weissman | Obtained from lab of Dr. Irv Weissman |
Riferimenti
- Shankman, L. S., et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 21, 628-637 (2015).
- Rowe, V. L., et al. Vascular smooth muscle cell apoptosis in aneurysmal, occlusive, and normal human aortas. J Vasc Surg. 31, 567-576 (2000).
- Rodella, L. F., et al. Abdominal aortic aneurysm and histological, clinical, radiological correlation. Acta Histochem. 118, 256-262 (2016).
- Lewandoski, M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2, 743-755 (2001).
- Lakso, M., et al. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 89, 6232-6236 (1992).
- Metzger, D., Clifford, J., Chiba, H., Chambon, P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 92, 6991-6995 (1995).
- Sheikh, A. Q., Lighthouse, J. K., Greif, D. M. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep. 6, 809-817 (2014).
- Greif, D. M., et al. Radial construction of an arterial wall. Dev Cell. 23, 482-493 (2012).
- Misra, A., et al. Integrin beta3 inhibition is a therapeutic strategy for supravalvular aortic stenosis. J Exp Med. 213, 451-463 (2016).
- Sheikh, A. Q., Misra, A., Rosas, I. O., Adams, R. H., Greif, D. M. Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med. 7, (2015).
- Wirth, A., et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 14, 64-68 (2008).
- Wendling, O., Bornert, J. M., Chambon, P., Metzger, D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 47, 14-18 (2009).
- Badea, T. C., Wang, Y., Nathans, J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 23, 2314-2322 (2003).
- Snippert, H. J., et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 143, 134-144 (2010).
- Kumar, M. E., et al. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science. 346, 1258810 (2014).
- Srinivas, S., et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 1, 4 (2001).
- Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L., Luo, L. A global double-fluorescent Cre reporter mouse. Genesis. 45, 593-605 (2007).
- Li, D. Y., et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 393, 276-280 (1998).
- Miano, J. M., Cserjesi, P., Ligon, K. L., Periasamy, M., Olson, E. N. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 75, 803-812 (1994).
- Curran, M. E., Atkinson, D. L., Ewart, A. K., Morris, C. A., Leppert, M. F., Keating, M. T. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 73, 159-168 (1993).
- Li, D. Y., et al. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 102, 1783-1787 (1998).
- Li, D. Y., et al. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 6, 1021-1028 (1997).
- Pober, B. R. Williams-Beuren syndrome. N Engl J Med. 362, 239-252 (2010).
- Pober, B. R., Johnson, M., Urban, Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest. 118, 1606-1615 (2008).
- Holtwick, R., et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 99, 7142-7147 (2002).
- Boucher, P., Gotthardt, M., Li, W. P., Anderson, R. G., Herz, J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 300, 329-332 (2003).
- Zhang, J., et al. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 26, 23-24 (2006).
- Armstrong, J. J., Larina, I. V., Dickinson, M. E., Zimmer, W. E., Hirschi, K. K. Characterization of bacterial artificial chromosome transgenic mice expressing mCherry fluorescent protein substituted for the murine smooth muscle alpha-actin gene. Genesis. 48, 457-463 (2010).
- Magness, S. T., Bataller, R., Yang, L., Brenner, D. A. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 40, 1151-1159 (2004).
- Yokota, T., et al. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells. 24, 13-22 (2006).

