Development of New Methods for Quantifying Fish Density Using Underwater Stereo-video Tools
Summary
We describe a new method for counting fishes, and estimating relative abundance (MaxN) and fish density using rotating stereo-video camera systems. We also demonstrate how to use distance from camera (Z distance) to estimate species-specific detectability.
Abstract
The use of video camera systems in ecological studies of fish continues to gain traction as a viable, non-extractive method of measuring fish lengths and estimating fish abundance. We developed and implemented a rotating stereo-video camera tool that covers a full 360 degrees of sampling, which maximizes sampling effort compared to stationary camera tools. A variety of studies have detailed the ability of static, stereo-camera systems to obtain highly accurate and precise measurements of fish; the focus here was on the development of methodological approaches to quantify fish density using rotating camera systems. The first approach was to develop a modification of the metric MaxN, which typically is a conservative count of the minimum number of fish observed on a given camera survey. We redefine MaxN to be the maximum number of fish observed in any given rotation of the camera system. When precautions are taken to avoid double counting, this method for MaxN may more accurately reflect true abundance than that obtained from a fixed camera. Secondly, because stereo-video allows fish to be mapped in three-dimensional space, precise estimates of the distance-from-camera can be obtained for each fish. By using the 95% percentile of the observed distance from camera to establish species-specific areas surveyed, we account for differences in detectability among species while avoiding diluting density estimates by using the maximum distance a species was observed. Accounting for this range of detectability is critical to accurately estimate fish abundances. This methodology will facilitate the integration of rotating stereo-video tools in both applied science and management contexts.
Introduction
Along the U.S. Pacific Coast, many of the species important to commercial and recreational groundfish fisheries (e.g., the rockfish complex (Sebastes spp.) and Lingcod (Ophiodon elongatus)) are strongly associated with high-relief, hard-bottom habitats1,2,3,4,5. Stereo-video drop cameras are an attractive non-extractive tool to use in rocky habitats due to the relative ease and simplicity of operation. A variety of stereo-video camera systems have been developed and deployed in southern-hemisphere, shallow-water ecosystems6,7,8,9,10, and recently, video drop-cameras have gained traction as a management tool for deep water rocky-reef environments along the Pacific Coast11,12,13. We sought to modify these existing stereo-camera designs by using a stereo-video camera system (hereafter referred to as "Lander") to more efficiently characterize fish populations in high-relief seafloors along the central Pacific Coast (see Table of Materials). The Lander used was different than existing video systems because cameras were mounted to a central rotating bar, which allowed for a full 360° of coverage of the seafloor at the drop location14. The Lander completed one full rotation per minute, which allowed us to rapidly characterize the abundance and community composition of an area and achieve the same level of statistical power with fewer Lander deployments. (See Starr (2016)14 for greater detail on the specifics of the Lander configuration). Preliminary tests in the study system suggested that eight rotations of the cameras in our surveys were sufficient to characterize species abundance and richness. This determination was made by an observation of diminishing returns in species abundance and fish density over longer drops. We recommend that a pilot study including longer soak times be conducted in any new system to determine the optimal soak time for a given ecosystem/study species.
By using paired stereo cameras, both total survey area and absolute fish density can be calculated for each video survey; however, the use of rotating cameras necessitated the modification of traditional fish count metrics. Stationary video systems most often use "MaxN" as a conservative count of fishes on a deployment6,10. Traditional MaxN describes the maximum number of fish of a given species observed together in a single video frame, in order to avoid double counting a fish that has left and returned to frame. MaxN has therefore been an estimate of the minimum number of fish known to be present and may underestimate true fish abundance6,10. The MaxN metric was redefined to represent the greatest number of fish seen in each full rotation of the cameras.
The second modification to previous stereo video methods was to account for the fact that species of various sizes, color, and shapes have different maximum distances of reliable identification. For example, large species such as O. elongatus have a distinct elongated shape and can reliably be identified at much greater distances compared with small and cryptic species such as the Squarespot Rockfish (Sebastes hopkinsi). These different maximum ranges of detectability change the effective area sampled by the Lander for each species. Because the stereo cameras allow us to place every fish in three-dimensional space with a high degree of accuracy, one can determine the distance from the cameras that each fish was measured (i.e., the "Z distance", named for the "z-axis" which is perpendicular to the straight line drawn between the cameras). For each species, the distance within which 95% of all individuals were observed (hereafter "95% Z distance") was considered to be the radius of the survey area, and was used to calculate the total area surveyed. In addition to species-specific characteristics, identifiability will be impacted by environmental conditions such as water turbidity. Because these factors can vary in time and space, it is important to use the 95% Z statistic only in aggregate. While it will be highly accurate for large samples, any one individual survey may vary in area surveyed.
The protocol detailed below provides guidance on how to create and use these metrics. Though the focus was to characterize deep-water rocky habitat along the Pacific Coast, the methodology described for modified MaxN count is readily applicable to any rotating drop-camera system. The number of camera rotations needed to characterize fish populations will depend on local ecosystem dynamics, but the conceptualization of the modified MaxN will remain the same. Similarly, whereas we used 3D photogrammetric software to analyze stereo video, the techniques described herein are easily applied across software platforms, as long as the precise location of fish in three-dimensional space is possible. Additionally, the approach of applying a 95% Z distance value could be considered in future studies with stereo-cameras to account for species-specific ranges of detectability and to more accurately calculate fish abundance.
Protocol
NOTE: Screenshots of software steps are included as Supplementary Files. Please note that the software steps described below are specific to the chosen software (see the Table of Materials). The overall approach can be extended to any stereo software platform.
1. Prepare Stereo-camera Footage for Analysis
NOTE: Calibration using a calibration cube is recommended. A calibration cube is a three-dimensional aluminum-frame with precisely positioned reflective dots on the surface. When used in conjunction with calibration software, a calibration cube leads to greater precision and accuracy than checkerboard approaches9.
- Calibrate the Lander cameras with stereo-calibration software (Figure 1 and Figure 2; see Table of Materials for a software recommendation).
NOTE: Calibration can be verified before use in the field by measuring targets of known sizes at varying distances (see Supplementary Video 1). Average measurement error for a 50-cm target at distances of 3 m (or less) should be within 2% of the known target length. Also note that a given calibration will only be valid if camera positions do not change relative to one another. It is critical to take care and avoid unintended jostling of the cameras until all sampling has been performed. - Collect field data using the calibrated Lander (Figure 1, Supplementary Video 2).
- After field study is complete, create a new project folder containing both video and calibration files.
NOTE: In each project folder there needs to minimum of five files: the left and right ".Cam" calibration files, the left and right video files (.MP4 or.AVI format only), and the species list (.txt format). - In the stereo measurement software, start a new measurement project, and load appropriate video and calibration files.
NOTE: Screenshots of all software steps are available among the Supplementary Files.- Navigate to 'Measurement' > 'New measurement file'.
- Set the picture directory by navigating to 'Picture' > 'Set picture directory', and choose the folder containing all project files.
- Load the left camera ".Cam" file by navigating to 'Stereo' > 'Cameras' > 'Left' > 'Load camera file' and selecting the appropriate file.
- Repeat step 1.4.3 to load the right camera ".Cam" file
NOTE: These files contain calibration measurements for each camera (e.g., pixel size, aspect ratio, radial distortion, decentring distortion, etc.) and will be used to measure fish and calculate distance-from-camera (i.e., Z distance). - Define the movie sequence for the left video file by navigating to 'Picture' > 'define movie sequence' and selecting the left camera video file.
- Load the left video file into measurement software by selecting 'Picture' > 'load picture'.
NOTE: Be sure that the 'Lock' box is unchecked before loading video files. This allows both videos to be loaded simultaneously. - Define movie sequence and load video file for the right video using the menus 'Stereo' > 'picture' > 'define movie sequence' and 'Stereo' > 'picture' > 'load picture'.
- Load the species list by navigating to 'Measurement' > 'Attributes' > 'Edit/load species file'.
- Enter survey ID information under 'Information Fields' > 'Edit field value' and save file to create an.EMObs project.
- Sync the videos using light flash, handclap, Coordinated Universal Time (UTC) stamp, or any time specific event that occurs in both videos.
- If using UTC time stamp, frame-step forward in the left video until the time stamp starts a new second. Else frame forward until light flash or handclap occurs.
- Frame-step the right video forward until the time stamp matches the left video exactly. Else frame step forward until the exact moment the light flash or handclap matches the left video.
NOTE: It is important that videos be synchronized to the same frame. Video synchronization should be checked periodically using the video time stamp to avoid camera frame drift during analysis. A filmed hand clap could also be used at the beginning and end of the video to test that right and left videos were synced to the same frame.
- Click the "Lock" button to ensure videos play together and maintain synchronization.
2. Generate Point Counts and Calculate MaxN
NOTE: Each fish is initially marked with a 2D point to the lowest possible taxonomic resolution. Fish with uncertain ID should be marked for later review.
- Wait to begin counting fish until the end of a complete camera rotation to ensure that a full 360 degrees is used. Also wait until sediment has cleared (generally <1 – 2 min after contact with bottom).
- As soon as the Lander starts its first rotation, right click to define a new sample period: 'Period definitions' > 'Add new start period'. Enter first period name as "01" and click "OK".
- As the Lander rotates, begin marking each fish that comes into frame with a 2D point using the left camera only.
- To add a 2D point, right click, select 'Add point', and choose the correct species name. Label to the lowest possible taxonomic level, selecting 'spp.' for unknown species and click "OK".
- Continue to mark each new fish according to step 2.2.1 until the conclusion of the rotation.
- Repeat protocol procedures 2.1 – 2.2 for each additional Lander rotation – ensuring that a new period is defined at the start of each camera rotation.
NOTE: Species accumulation curves were used to determine that eight rotations were, on average, sufficient to characterize fish abundance in the present study. Researchers should consider conducting preliminary tests with additional camera rotations, over longer soak times, to characterize the optimum number of camera rotations within a particular ecosystem. - Calculate species-specific counts of individuals observed per camera rotation.
- After all rotations have been enumerated, export 2D points by navigating to 'Measurement' > 'Measurement summaries' > 'Point measurements' and save 2D points as a.txt file.
- Open the saved 2D.txt point file as a spreadsheet and create a PivotTable of species vs. rotation number to summarize counts (Table 1) by navigating to 'Insert' > 'PivotTable'. Select "Genus and Species" for 'Row Label', and "Period" for 'Column Label'.
- Choose the MaxN for each species by selecting the camera rotation that has the greatest number of individuals of that species (Table 1).
- For fishes identified only to genus, select a genus-level MaxN based on the rotation that had the greatest number of individuals identified to species in that particular genus.
NOTE: This step helps to avoid double-counting individual fish that were only identifiable to higher taxonomic groups (e.g., only to genus or family). For example, in Table 1, 'rotation 1' contained 10 unidentified Sebastes spp. and 33 members of the genus Sebastes identified to species, whereas 'rotation 3' contained only two unidentified Sebastes spp. and 43 members of the genus Sebastes identified to species. Therefore 'rotation 3' would be used for MaxN count of unknown Sebastes spp. In this way, the conservative assumption is made that 8 of the unidentified Sebastes spp. in 'rotation 1' were identified in 'rotation 8'. - If multiple rotations have the same MaxN count for a given species, choose the first rotation with MaxN for 3D point measurements.
- For each species, take 3D measurements of fish in the rotation that MaxN occurred.
- Use the saved 2D points collected in steps 2.1 – 2.3 to navigate to the exact same fish for 3D measurement.
- Zoom in at least 4X to better identify the tip of the fish snout and edges of caudal fins (Figure 3).
NOTE: It may be necessary to frame step forward or backward to find the best orientation of the fish for a 3D measurement. The 'best' orientation is one where both the snout and edges of the caudal fins are visible in both cameras. - Manually click on the tip of the snout, then the edge of the tail in the left camera, then repeat the selection in the same order in the right video.
- Select correct species identification from dropdown menus as was done in 2.2.1.
- If a 3D length measurement is not possible, for instance if the head and tail of the fish are not visible in both cameras, then mark a 3D point instead by left clicking the same position of the fish in both the left and right videos. Fill out the information fields as before and leave the comment "Exclude from length measurement".
NOTE: MaxN may occur on different rotations of the cameras for different species; however, for any given species, measurements should occur in one rotation only (Table 1).
- After completing 3D measurements for all fishes, export data as.txt file for further analysis.
- Navigate to 'Measurement' > 'Measurement summaries' > '3D Point and length measurements', and save.txt file to export.
3. 95% Z distance procedure for species-specific survey areas
NOTE: The 95% Z distance is an estimate of the average distance a species could reliably be identified in a given study while excluding cases of exceptional conditions of water clarity or lighting. This calculation takes into account the average oceanographic conditions for a given study and will need to be re-calculated for each new study.
- Use simple bootstrapping to determine if the sample size is great enough to characterize the distance of reliable detection for each species.
- For each sample size class (e.g., sample size bins of 5 fish), take 1,000 random draws of the selected sample size with replacement from the sample population and calculate the mean 95% quantile of distances of these 1,000 draws, and plot the resulting asymptotic curve. See supplied code in Supplemental Files 1 & 2.
- Verify that adequate samples were obtained by comparing the actual sample size with the 95% Z distance asymptote with increasing sample size.
- Calculate the 95% Z distance value as the 95% quantile of distance-from-camera measured for a species across all surveys.
- Calculate the effective area surveyed for each species using the 95% Z value.
NOTE: In the case of a rotating Lander, the 95% Z value represents the outer radius of a surveyed swath, with the inner radius determined by the physical setup of the tool and how close to the base the cameras are able to observe. As the Lander rotates, a 'donut' shaped survey area is formed (Figure 4).- Calculate area surveyed as:
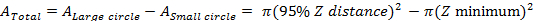
NOTE:For example, a relatively large species like Yelloweye Rockfish (Sebastes ruberrimus) had a 95% Z distance of 3.3 m and an effective survey area of 30.9 m2 per Lander deployment: 34.3 m2 (outer circle) – 3.4 m2 (inner circle) = 30.9 m2 (total survey area).
- Calculate area surveyed as:
- Using the calculated area surveyed (step 3.3.1), convert individual species counts (MaxN) into density estimates for each visual survey using the equation:
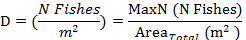
NOTE: A similar procedure could be used to calculate a volumetric density rather than an areal density; however, that process is not described here.
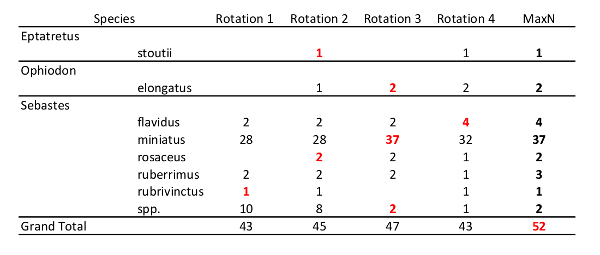
Table 1: Example MaxN summary table. The selection of MaxN for each species is demonstrated with red and bold text. Note that a conservative MaxN for unidentified Sebastes spp. was determined by the rotation with the most Sebastes identified to species (rotation 3). Also, while this study used eight camera rotations, only four rotations are displayed in Table 1 for simplicity. The process for selecting MaxN is identical regardless of the number of rotations.
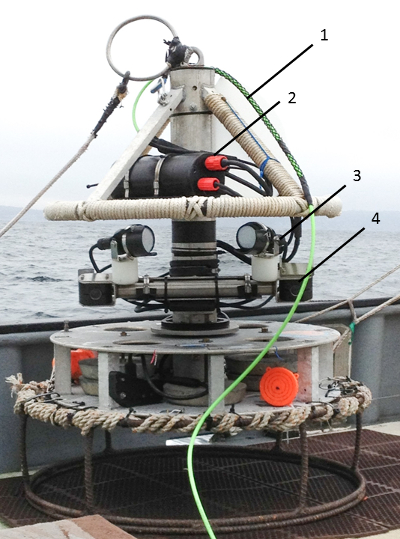
Figure 1: Stereo video Lander. Key hardware is numbered (1) 300 m umbilical, (2) two digital video recorders (DVR) with removable 32GB storage cards inside waterproof bottle, (3) two LED lights outputting 3,000 lumens at a color temperature of 5,000 K, and (4) two cameras with 620 TV line (TVL) resolution. Please click here to view a larger version of this figure.
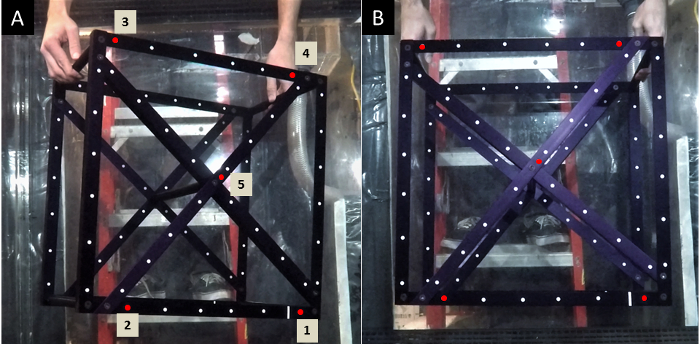
Figure 2: Calibration cube (500 mm x 500 mm x 300 mm). Example of a calibration with a 'calibration cube' shown in two different orientations: (A) the right side of the cube is pushed out towards cameras, and (B) the face of the cube is parallel to the face of the cameras. Red dots denote the reference points used in this particular calibration method and must always be identified in the numbered order. Please click here to view a larger version of this figure.

Figure 3: 3D measurement placed on Sebastes miniatus. The tip of the snout and end of the tail were identified in each camera frame to allow for stereo measurement. Please click here to view a larger version of this figure.
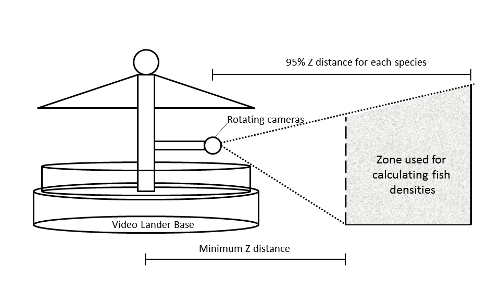
Figure 4: Area surveyed by the Lander tool. Effective area surveyed by the Lander tool was bounded by the minimum Z distance, and the 95% Z distance for each species. Note that this area created a 'donut' shaped survey volume around the Lander. Please click here to view a larger version of this figure.
Representative Results
Between 2013 and 2014, we conducted 816 surveys with the rotating stereo-video Lander (Figure 1) along the central California coast and collected MaxN and 95% Z distance (Figure 4) data on more than 20 species. There were clear patterns in the effective detectable range of species observed, likely due to the interaction of species' size, shape, and coloration (Figure 5). For instance, the Flag Rockfish (Sebastes rubrivinctus) has distinct banding on its sides allowing for confident identification at greater distances than other species of comparable size. Similarly, Canary Rockfish (Sebastes pinniger) are relatively large bodied, but have a pigmentation that is similar to other species, thus making it more difficult to identify at distance (Figure 5).
We use two species to demonstrate the calculations of both MaxN and 95% Z distance values: Pygmy Rockfish (Sebastes wilsoni) and Lingcod (O. elongatus). The former is a small-bodied fish that can be difficult to identify at distance; whereas O. elongatus is relatively large, has a distinct shape, and is more easily identifiable. From 2013 – 2014, 1,191 measurements for S. wilsoni and 1,222 measurements for O. elongatus were collected. Then, the 95% quantiles of distances at which these species were observed: the 95% Z distances were 2.65 m for S. wilsoni and 3.96 m for O. elongatus (Figure 5) were calculated. These 95% Z distances translate into effective survey areas of 18.6 m2 and 46.0 m2 for S. wilsoni and O. elongatus, respectively. A simple bootstrap analysis confirmed that sufficient sample sizes were obtained for characterization of 95% Z distance values. For both species, the estimate of 95% Z distance stabilized when greater than 50 surveys containing these species were sampled, providing strong evidence that the chosen sample sizes were more than adequate to characterize the effective Lander sample area for these species (Figure 6).
MaxN counts per survey were then converted into densities (number of fish/m2). We used density estimates from the 816 surveys to test the hypothesis that Lingcod and Pygmy Rockfish would be observed primarily on high relief habitats. For both species, there were significantly greater densities over high and medium relief compared with low relief habitats (Kruskal-Wallis, p <<.001; Figure 7). These results were consistent with previously reported habitat associations for both species15. There were no differences between medium and high relief habitat for either species.
To understand how the rotating Lander compared with traditional stationary camera systems, we estimated differences in density and variability estimates between a rotating and a simulated stationary Lander. We assumed a typical stationary single-camera Lander would have a 90-degree field of view. The rotating Lander has a 60-degree field of view, and requires 5 seconds of rotation to complete a 90-degree view. Using 261 surveys, we selected fish observation data from the middle 5 seconds of Lander rotations to establish MaxN. Density estimates for the pseudo-stationary Lander were standardized by using the reduced areas of coverage (i.e., approximately ¼ the area of the rotating Lander). Differences in mean density and coefficient of variation between rotating and pseudo-stationary Landers were evaluated with Welch's t-test. Mean densities obtained by the rotating camera were 18% greater than those obtained with stationary cameras (Welch's t21.7, p = 0.081, Figure 8A). Additionally, the coefficient of variation was 1.8 times greater with the stationary camera compared to rotating cameras (Welch's t15.1, p <0.001, Figure 8).
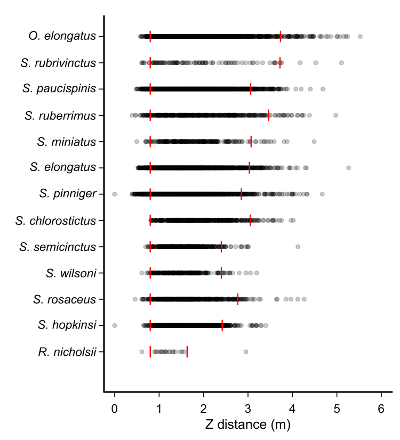
Figure 5: Z distances observed for select species. Red vertical bars denote the minimum Z distance (0.81 m from cameras) on the left and the 95% Z Distance value on the right. Note that this represents the average effective survey area around the Lander for each species. Please click here to view a larger version of this figure.
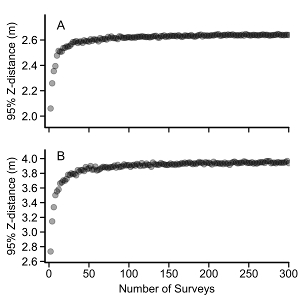
Figure 6: Bootstrapped Z distance values. Bootstrapping to increase the sample size for (A) S. wilsoni and (B) O. elongatus observations. Sample sizes ranging from 3 – 300 were bootstrapped 1,000 times each to calculate the mean 95% Z distance and verify the sample sizes were adequate. Note that the y-axis values range from 2.0 – 2.6 m for S. wilsoni and from 2.6 – 4.0 m for O. elongatus. Please click here to view a larger version of this figure.
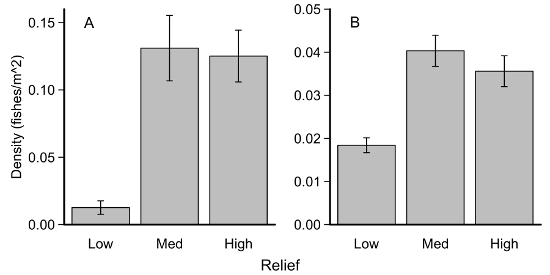
Figure 7: Habitat differences for two select species. Average densities (± SE) of (A) S. wilsoni and (B) O. elongatus measured on low, medium, and high relief rock habitat. Please click here to view a larger version of this figure.
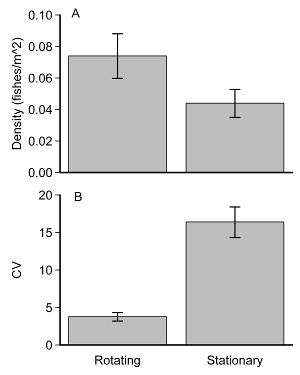
Figure 8: Differences between rotating and pseudo-stationary landers. Both estimates of (A) mean density (fishes/m2 ± SE) and (B) mean coefficient of variation (CV) ± SE for 261 surveys are presented. Please click here to view a larger version of this figure.
Supplementary Video 1: Calibration verification. Calibration can be verified before use in the field by measuring targets of known sizes at varying distances. Please click here to view this video. (Right-click to download.)

Supplementary Video 2: Underwater Survey Footage. Please click here to view this video. (Right-click to download.)
Discussion
The traditional MaxN metric is predicated on the idea of counting a guaranteed minimum number of individuals present during a survey. If a certain number of fish are simultaneously visible in a single video frame, there cannot be any fewer present, but because fish are mobile and heterogeneously distributed, the likelihood of seeing all individuals simultaneously during a single video frame is low. It is therefore likely that traditional MaxN underestimates true fish abundance16,17. Additionally, it has been demonstrated that traditional MaxN may display non-linear negatively-biased relationships with increasing fish abundances16,18. This may be related to the phenomenon of gear saturation whereby relative abundance indices fail to detect true increases in abundance19,20. Conversely, the apparent stability of an index with truly declining fish abundance has been termed 'hyperstability', and may ultimately lead to the crash of fish populations21,22. A recent study reported that instability in MaxN could be alleviated by increasing the surveyed field of view16. In that study, the relationship between MaxN and true abundance became increasingly linear as the field of view approached 100% (i.e., 360 degrees).
The results from the stationary camera simulation indicate congruence with these previous results, and suggest that the MaxN value may better characterize fish abundance. For example, the estimated mean coefficient of variance was reduced among density estimates derived from the rotating Lander compared with the pseudo-stationary Lander. This is likely due to the fact that fish are heterogeneously distributed, and that stationary cameras are more likely to 'miss' the fish present if the Lander faces the wrong direction. Rotating Landers maximize sampling effort by surveying the full 360 degrees around the tool, and the net effect is reductions to both sampling cost and variance, and an overall increase in the statistical power of the study. Future studies could better address this issue by directly testing a rotating Lander with a separate stationary Lander in a paired survey design. Similarly, we were unable to directly test for the relationship between MaxN and true abundance in this study, and future studies might directly test this using either simulation or controlled environments, as was done in Campbell (2015)16.
A possible criticism of the modified MaxN approach is the possibility of double counting individuals. Because the Lander made one full rotation per minute, and the benthic species of interest in the ecosystem tend to be relatively sedentary and slow moving under most conditions, we believe the risk of double counting was low. Additionally, cases where fish would enter or leave the survey area over the course of the eight rotations were observed. Additional precautions to avoid double counting such as using the rotation with the greatest number of individuals of a given Genus to count unidentified species were taken. Other metrics have been proposed as indices of fish abundance such as Mean Count; however, these too have been shown to consistently underestimate true abundance while increasing variability among density estimates16. MaxN is therefore recommended as a more precise metric of fish abundance. While our modified MaxN metric does not guarantee a conservative estimate of absolute minimum number of individuals, we are overall confident that this modified MaxN approach provides better estimates of true fish abundance, and that over-counting fish is of relatively low concern.
Many side-viewing video-transect surveys use a fixed transect width to estimate density for all species. Similarly, one approach to using stereo-video Landers would be to use one maximum distance-from-camera to calculate both area surveyed and fish density. Both may lead to an underestimate of species which are only reliably identifiable to smaller distances than the fixed transect width estimates23. The distance to which a species is reliably identified is caused by the interaction of factors such as size, shape, coloration pattern, fish behavior, as well as environmental factors. The 95% Z distance method is particularly advantageous in that it accounts for the interaction of all these factors simultaneously. For example, O. elongatus was the species that we are able to identify to the greatest distance, likely as a result of its distinct, large, elongate body shape and behavioral tendency to lay on the seafloor. Rosy Rockfish (Sebastes rosaceus) had one of the shortest Z distances, likely because, as a member of the Sebastomus subgenus, it has several congeners that look very similar and are difficult to distinguish at increased distances. By allowing for species-specific areas surveyed by the Lander, we may be able to more accurately estimate fish abundance. The bootstrap approach to sample size verification is simple and readily implemented in other surveys, and we believe the method of 95% Z distance could be further adapted to accommodate line transect survey design. 95% Z distance would then represent a horizontal distance of reliable detection for species observed with submersible or remotely operated vehicle (ROV) tools. In the future, researchers may investigate using distance sampling theory to model density as a function of detectability with distance23,24.
As there is greater use of no-take reserves in fisheries management25,26,27, there is an increasing need for non-extractive sampling techniques, especially in deep water habitats not accessible to diver surveys. However, it is also necessary that those techniques provide accurate, reliable data on fish length, abundance, and species composition. Video Landers are a relatively new monitoring tool that have a low cost, can be operated on relatively small vessels of opportunity, and are logistically simpler to operate than ROVs and submersibles while requiring fewer and less skilled personnel. While not discussed in these methods, stereo-camera Landers are capable of accurate length measurements with error less than 2%. Additionally, Landers can be rapidly deployed over large geographic areas, increasing statistical inference. We expect the interest in video monitoring tools to increase as research agencies look to tighten budgets and more efficiently spread sampling effort. Our modification of MaxN and 95% Z distance should be considered in future ecological studies utilizing rotating video Landers.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was funded by The Nature Conservancy and private donors, Resources Legacy Fund Foundation, Gordon and Betty Moore Foundation, Environmental Defense Fund, California Sea Grant Program, the NMFS National Cooperative Research Program, and a NOAA Saltonstall-Kennedy Grant #13-SWR-008. Marine Applied Research and Exploration (Dirk Rosen, Rick Botman, Andy Lauerman, and David Jefferies) developed, constructed and maintained the video Lander tool. We thank Jim Seager and SeaGIS™ software for technical support. Captain and commercial fisherman Tim Maricich and crew onboard the F/V Donna Kathleen provided support in deploying the Lander from 2012-2015. Thank you to all who participated in video data collection or analysis (Anne Tagini, Donna Kline, Lt. Amber Payne, Bryon Downey, Marisa Ponte, Rebecca Miller, Matt Merrifield, Walter Heady, Steve Rienecke, EJ Dick, and John Field).
Materials
| calibration cube | SeaGIS | http://www.seagis.com.au/hardware.html | 1000x1000x500 mm is the preferred dimensions. Other methods of calibration are available. |
| CAL calibration software | SeaGIS | http://www.seagis.com.au/bundle.html | |
| EventMeasure stereo measurement software | SeaGIS | http://www.seagis.com.au/event.html | |
| Statistical software | R Core Team 2017 (v. 3.4.0) | Bootstrapping code can be found: https://github.com/rfields2017/JoVE-Bootstrap-Function | |
| Spreadsheet Software | Microsoft Excel | ||
| 2 waterproof cameras | Deep Sea Power and Light | HD quality preferred | |
| 2 depth rated, waterproof lights | Deep Sea Power and Light : 3000 lumen LED with 5000k color temperature | ||
| DVR recorder | Stack LTD DVR | ||
| standard PC | Windows 10 preferred OS | ||
| rotating Lander platform | Marine Applied Research and Engineering (MARE) |
Riferimenti
- Love, M. S., Yoklavich, M. M., Thorsteinson, L. K. . The Rockfishes of the Northeast Pacific. , (2002).
- Laidig, T. E., Watters, D. L., Yoklavich, M. M. Demersal fish and habitat associations from visual surveys on the central California shelf. Estuar. Coast. Shelf Sci. 83 (4), 629-637 (2009).
- Anderson, T. J., Yoklavich, M. M. Multiscale habitat associations of deepwater demersal fishes off central California. Fish. Bull. 105 (2), 168-179 (2007).
- Yoklavich, M. M., Cailliet, G. M., Sullivan, D. E., Lea, R. N., Love, M. S. Habitat associations deep-water rockfishes a submarine canyon an example of a natural refuge. Fish. Bull. 98 (3), 625-641 (2000).
- . . Status of the Pacific Coast Groundfish Fishery, Stock Assessment and Fishery Evaluation. , (2016).
- Cappo, M., Harvey, E., Malcolm, H., Speare, P., Beumer, J. P., Grant, A., Smith, D. C. Potential of video techniques to monitor diversity, abundance and size of fish in studies of marine protected areas. Aquatic protected areas- What works best and how do we know. , 455-464 (2003).
- McLean, D. L., Green, M., Harvey, E. S., Williams, A., Daley, R., Graham, K. J. Comparison of baited longlines and baited underwater cameras for assessing the composition of continental slope deepwater fish assemblages off southeast Australia. Deep-Sea Research Part I: Oceanographic Research Papers. 98, 10-20 (2015).
- Parker, D., Winker, H., et al. Insights from baited video sampling of temperate reef fishes: How biased are angling surveys. Fish. Res. 179, 191-201 (2016).
- Boutros, N., Shortis, M. R., Harvey, E. S. A comparison of calibration methods and system configurations of underwater stereo-video systems for applications in marine ecology. Limnol. Oceanogr. Methodss. 13 (5), 224-236 (2015).
- Harvey, E. S., Cappo, M., Butler, J. J., Hall, N., Kendrick, G. A. Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar. Ecol. Prog. Ser. 350, 245-254 (2007).
- Watson, J. L., Huntington, B. E. Assessing the performance of a cost-effective video lander for estimating relative abundance and diversity of nearshore fish assemblages. J. Exp. Mar. Bio. Ecol. 483, 104-111 (2016).
- Easton, R. R., Heppell, S. S., Hannah, R. W. Quantification of Habitat and Community Relationships among Nearshore Temperate Fishes Through Analysis of Drop Camera Video. Mar. Coast. Fish. 7 (1), 87-102 (2015).
- Hannah, R. W., Blume, M. T. O. Tests of an experimental unbaited video lander as a marine fish survey tool for high-relief deepwater rocky reefs. J. Exp. Mar. Bio. Ecol. 430, 1-9 (2012).
- Starr, R. M., Gleason, M. G., et al. Targeting Abundant Fish Stocks while Avoiding Overfished Species: Video and Fishing Surveys to Inform Management after Long-Term Fishery Closures. Plos One. 11 (12), 0168645 (2016).
- Love, M. S. . Certainly more than you want to know about the fishes of the Pacific Coast: a postmodern experience. , (2011).
- Campbell, M. D., Pollack, A. G., Gledhill, C. T., Switzer, T. S., DeVries, D. A. Comparison of relative abundance indices calculated from two methods of generating video count data. Fish. Res. 170, 125-133 (2015).
- Cappo, M., Speare, P., De’ath, G. Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Bio. Ecol. 302 (2), 123-152 (2004).
- Schobernd, Z. H., Bacheler, N. M., Conn, P. B., Trenkel, V. Examining the utility of alternative video monitoring metrics for indexing reef fish abundance. Can. Jour. Fish. Aquat. Sci. 71 (3), 464-471 (2014).
- Hansen, M. J., Schorfhaar, R. G., Selgeby, J. H. Gill-Net Saturation by Lake Trout in Michigan Waters of Lake Superior. North Am. J. Fish. Manag. 18 (4), 847-853 (1998).
- Dauk, P. C., Schwarz, C. J. Catch estimation in the presence of declining catch rate due to gear saturation. Biometrics. 57 (1), 287-293 (2001).
- Hilborn, R., Walters, C. J. . Quantitative Fisheries Stock Assessment Choice, Dynamics and uncertainty. , (1992).
- Erisman, B. E., Allen, L. G., Claisse, J. T., Pondella, D. J., Miller, E. F., Murray, J. H. The illusion of plenty: hyperstability masks collapses in two recreational fisheries that target fish spawning aggregations. Can. Jour. Fish. Aquat. Sci. 68, 1705-1716 (2011).
- Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L. . Distance Sampling: Estimating abundance of biological populations. , (1993).
- Ronconi, R. A., Burger, A. E. Estimating seabird densities from vessel transects: Distance sampling and implications for strip transects. Aquat. Bio. 4 (3), 297-309 (2008).
- Caselle, J. E., Rassweiler, A., Hamilton, S. L., Warner, R. R. Recovery trajectories of kelp forest animals are rapid yet spatially variable across a network of temperate marine protected areas Recovery trajectories of kelp forest animals are rapid yet spatially variable across a network of temperate marine protected. Nat. Publ. Gr. , 1-14 (2015).
- Starr, R. M., Wendt, D. E., et al. Variation in Responses of Fishes across Multiple Reserves within a Network of Marine Protected Areas in Temperate Waters. Plos One. 10 (3), 0118502 (2015).
- Lester, S., Halpern, B., et al. Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33-46 (2009).

